Anatomy of pH Electrodes
Methods of pH Measurement
(Updated September 2021)
How do pH electrodes work? Visual, photometric, and potentiometric methods can be used to measure the hydrogen ion activity of a solution. Visual and photometric methods rely on color changes of specific organic pigments in order to determine pH. Visual methods are completed with visual indicators such as pH test strips, while photometric determination involves shining a light through the sample and measuring the absorbance.
The application of visual or photometric determination of pH is limited. Measurements will be unreliable if the solution to be measured is cloudy or has an inherent color. Some measurement solutions also contain chemical bonds which destroy the color indicators through oxidation or reduction and produce incorrect results.
Potentiometric methods determine pH by using the electrical potential of pH-sensitive electrodes as a measurement signal. The disadvantages of visual and photometric methods are not present with potentiometric methods of a pH probe. Potentiometric determination of pH can be used in almost any application, as potentiometric sensors are very sensitive and selective.
A distinction is made between hydrogen, metal (e.g. antimony), and glass electrodes, with the glass electrode being the most commonly used pH sensor.
How do pH probes work? The glass pH probe is an example of an ion selective electrode (ISE). This measuring system basically consists of the ISE reacting on a special ion type, in this case, the hydrogen ion, and a reference electrode that are jointly immersed in the sample to be measured. (Learn more, pH Measurement Principles and Best Measurement Practices | Webinar).
The hydrogen ISE provides an electrochemical potential that is influenced by the hydrogen ion activity of the solution. The reference electrode, however, is intended to build up an electrochemical potential that does not depend on the composition of the sample. The difference between these potentials, the voltage (mV) displayed on a pH meter, determines the pH value based on the Nernst equation.
Glass pH Electrode Structure and Design
A typical pH electrode consists of several unique structures, each of which can be seen in Figure 1. The principle components are the electrode body, pH-sensitive glass membrane, reference electrode (i.e. reference system), reference electrolyte, and the reference junction.
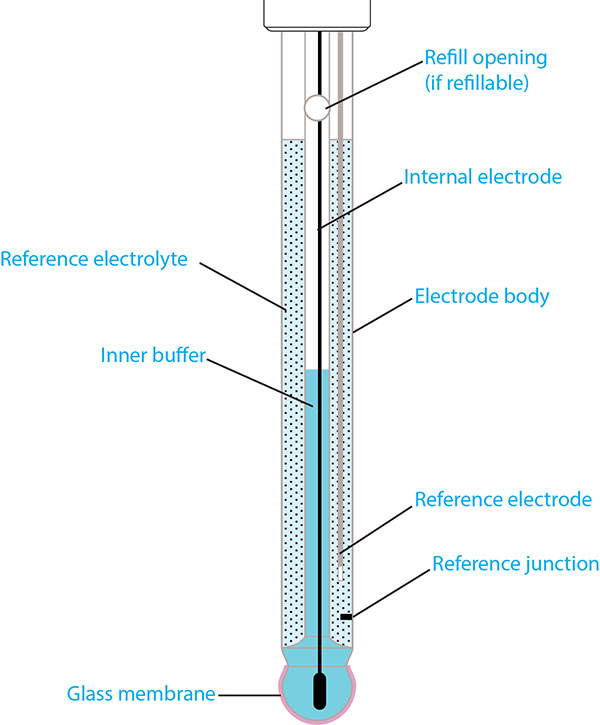
Figure 1: Structure of a typical pH electrode
Electrode Body
The term ‘glass electrode’ is not indicative of the material used to construct the electrode body, as electrodes can have either a plastic or glass electrode body. Rather, ‘glass electrode’ is used to describe the membrane material (i.e. glass membrane).
All YSI field electrodes are constructed from plastic, as plastic body electrodes are more rugged and less likely to crack than glass. YSI offers both glass and plastic body electrodes for the lab, with glass electrodes typically possessing a greater range of operating temperature.
YSI field pH probes are not always entirely plastic. The pH sensing module on ProDSS and EXO pH sensors are plastic, but the rest of the electrode body is constructed from titanium in order to ensure durability in the field. A ProDSS pH/ORP sensor can be seen in Figure 2.

Figure 2: YSI ProDSS pH/ORP sensor
Glass Membrane
With the glass electrode, a glass membrane is fused on as a pH sensor. This membrane is filled with a buffer solution of known pH (typically pH = 7). This electrode design creates an environment with constant binding of H+ ions on the inside of the glass membrane, while the outside of the glass membrane is exposed to the sample where a variable amount of H+ ions exist. The difference in H+ ions creates a potential that is read versus the stable potential of the reference electrode.
In order to ensure optimal moistening of the glass membrane, the membrane shape can vary. Sphere and cone membrane shapes can be used for most applications, but unique applications may require a specialized membrane, such as the spear tipped TruLine 21 for penetrating semi-solid media and the TruLine 27 with a flat membrane for surface measurements (Figure 3). Table 1 provides an overview of the different types of pH membranes. 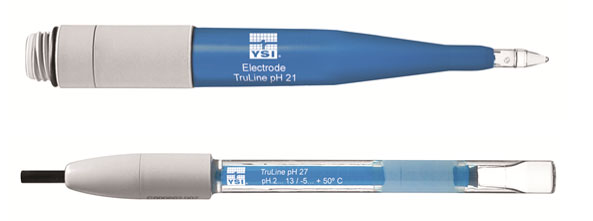
Figure 3: YSI TruLine 21 (spear tip membrane) and YSI TruLine 27 (flat membrane)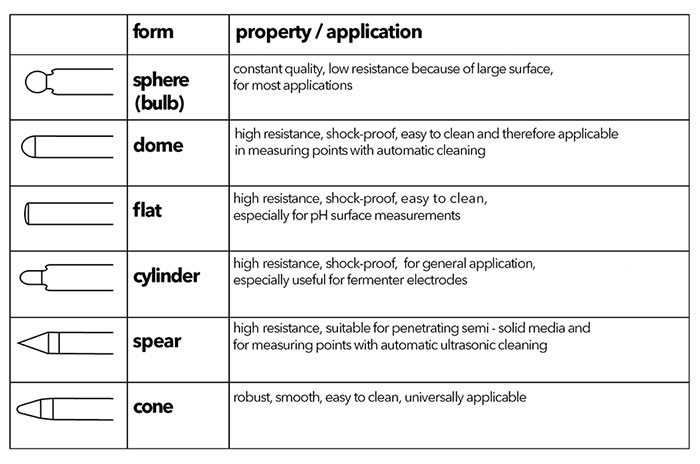
Table 1: Glass Membrane Shapes
Why Is a Reference Needed?
pH measurement with a glass pH electrode relies on the measurement of a voltage. In order to measure the voltage, two points with different electrical potential values are required. The reference electrode is designed to maintain a constant electrical potential that is independent of the sample composition and temperature. In contrast, the hydrogen ion selective electrode (ISE) with glass membrane provides an electrical potential that is dependent upon the activity of hydrogen (H+) ions in the sample solution. Therefore, both the reference electrode and the hydrogen ISE are needed when determining pH.
Types of Reference Systems
The reference electrode/system and the hydrogen ISE (i.e. the electrode with the glass membrane) can be separate electrodes (Figure 4) or they can be combined into a single electrode for convenience (Figure 1 and Figure 5). The combination-style electrode is very common and all YSI electrodes are of the combination type.
Regardless of the electrode design or the type of reference system used, the reference electrode is immersed in reference electrolyte (typically KCl). The reference electrolyte will be discussed in more detail.
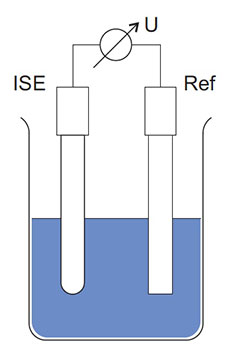
Figure 4: Schematic structure of a hydrogen ISE, reference, and voltmeter
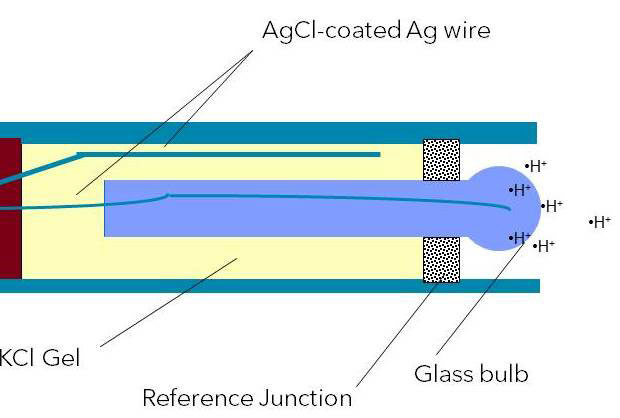
Figure 5: Combination pH electrode with KCl gel reference electrolyte
The most common type of reference electrode used today is the silver/silver chloride (Ag/AgCl) system. Since silver is non-toxic to humans, Ag/AgCl electrodes can also be used in medicine and food technology where poisonous mercury and thallium systems are prohibited. Disposal is also less critical with Ag/AgCl than with thallium and mercury. Ag/AgCl has a wide range of applications with respect to temperature (up to 140°C) and is therefore also suitable for sterilizable electrodes. Most YSI electrodes feature a Ag/AgCl reference system.
The mercury chloride (Hg/Hg2Cl2 ) system has been in use for the longest time. This reference system displays the most stable potential in the presence of KCl. However, today it is only used in exceptional cases due to the toxicity of mercury. Also, the reliable temperature range when using mercury chloride is relatively narrow. At temperatures above 60°C the Hg2Cl2 begins to degrade.
The iodine/iodide system, a relatively new reference system with a fast response time, has recently been developed. Compared to conventional electrodes with Ag/AgCl reference systems, electrodes with iodine/iodide reference systems have the advantage of much lower temperature sensitivity, as the temperature coefficient of this reference system is almost zero.
The iodine/iodide system is also metal ion free, a feature that is especially useful when measuring in Tris buffer and protein solutions. Reference systems with metal ions (e.g. Ag/AgCl) will interact with these solutions, ultimately resulting in the reference junction becoming clogged.
The advanced YSI IoLine series of pH electrodes feature an iodine/iodide reference system. The unique color of the IoLine reference can be seen in Figure 6.

Figure 6: YSI IoLine pH electrode
Properties of a Good Reference Electrolyte
The reference electrolyte has a connection to the sample through the junction, as it serves to close the electrical circuit in the electrode.
A good reference electrolyte must have certain qualities. The reference electrolyte must have good electrical conductivity and be chemically neutral. Since some electrolyte will typically leak into the sample during measurement, it is also important the electrolyte not react with the measurement solution.
The ions of an electrolyte solution must also be equally mobile. If ions in the electrolyte solution diffuse at different rates, an electrical potential (i.e. diffusion potential) can form due to the division between the positive and negative charge. This unwanted potential can be quite troublesome when measuring pH. With some ions (e.g. K+ and Cl-), the differences in diffusion speed are small, resulting in a much smaller diffusion potential.
Potassium chloride (KCl) possesses all of these qualities. As a result, KCl is the most commonly used electrolyte solution.
Form of Liquid Electrolyte
Electrodes can have a gel, polymer, or liquid electrolyte. All YSI field electrodes and some YSI lab electrodes (e.g. TruLine 25 and TruLine 26) feature a gel electrolyte, while most YSI lab electrodes feature a liquid electrolyte reference that can be refilled. Polymer electrolyte is used in specialized YSI electrodes with a flat tip (TruLine 27) and spear tip (TruLine 21).
Liquid Electrolyte
Electrodes with liquid electrolyte can typically be refilled, thus resulting in a longer electrode life. Therefore, unlike an electrode with gel electrolyte, the electrolyte can easily be drained and replaced if the electrolyte becomes contaminated.
If using a YSI lab electrode with refillable reference, it is important to remember the refilling opening must always be open during calibration and measurement! Also, ensure the fill level of the electrolyte is at least 2 cm above the level of the calibration and/or measurement solution.
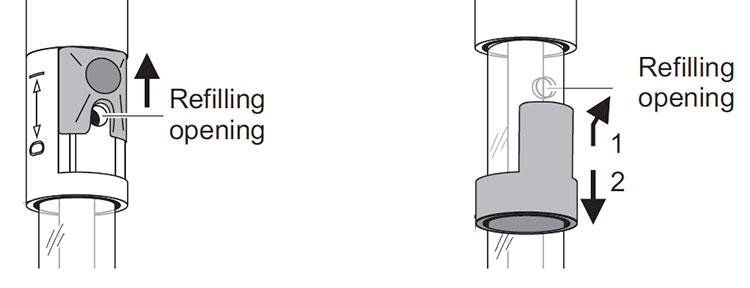
Figure 7: Slider (left) and stopper (right) refill openings for YSI lab electrodes
Response time is typically faster with electrodes featuring liquid electrolyte. Also, electrodes with liquid electrolyte have fewer limitations in their range of use, as gel and polymer electrolytes have a lower resistance to temperature and temperature changes. Unlike electrodes with liquid electrolyte, the incredibly small outflow rate of gel and polymer electrolyte in strongly acidic, basic, and low-ionic strength solutions can result in measurement errors due to the formation of diffusion potentials.
All YSI refillable lab electrodes utilize 3 M KCl for the reference electrolyte. However, since the electrolyte is connected to the sample via the junction, some applications require specialized electrolyte solutions.

Figure 8: YSI refillable lab electrodes (Science pHT-G and TruLine 17)
The YSI IoLine electrode design allows for the use of other electrolytes that meet the demands of the sampling application. For example, if electrolyte is needed that is completely chloride free (i.e. not KCl), another bridge electrolyte, such as 0.6 M potassium sulfate (K2SO4), can be used with the IoLine.
Gel and Polymer Electrolyte
Gel electrolyte still consists of KCl, but a gelling agent is added in order to prevent the electrolyte from readily leaking into the sample via the reference junction during measurement. Since there is virtually no loss of the electrolyte, these electrodes are easier to maintain, as there is no need to refill them with electrolyte. Since they cannot be refilled, electrodes with a gel electrolyte have a shorter lifetime than those with a liquid electrolyte.

Figure 9: YSI TruLine pH 25 electrode with gel electrolyte and plastic body
Polymer electrolyte is solid and can directly contact the sample during measurement. Due to the absence of electrolyte outflow, the mobility of all ions is greatly limited. This results in no precipitation of silver at the junction and makes the diffusion of foreign ions into the electrode virtually impossible.
The specialized YSI TruLine 21 and 27 pH electrodes feature the REFERID system, a unique form of polymer electrolyte. These electrodes are low maintenance and are characterized by high resistance to pressure and pressure changes. The REFERID system also features a visible KCl reserve.
pH Reference Junction, What’s Your Function?
The reference junction, also known as a diaphragm, creates electrical contact between the reference system and the solution. Much like the reference electrolyte, the reference junction must possess certain qualities.
Diffusion voltages at the junction are a common measurement error, so the junction plays a major role in the precision of measurements. To keep these disruptive potentials small, the junction must guarantee a relatively large and consistent outflow of reference electrolyte. However, the junction must only be slightly permeable to prevent electrolyte from escaping too quickly, which is especially important with electrodes utilizing liquid electrolyte. Different junction types have different outflow rates of electrolyte.
In addition to the permeability of the junction, its electrical resistance should be as low as possible and it must be chemically inert.
Types of Reference Junctions
There are several types of junctions, each with unique characteristics. Depending on the application, a decision must be made for YSI electrodes with liquid electrolytes as to whether a ceramic, ground-joint, or platinum junction best fits the measurement conditions. Table 1 provides an overview of the different junction types, including the annular gap (TruLine 27) and fiber (TruLine 25 and TruLine 26, MultiLab IDS 4110) junctions.
Table 2: Types of reference junctions
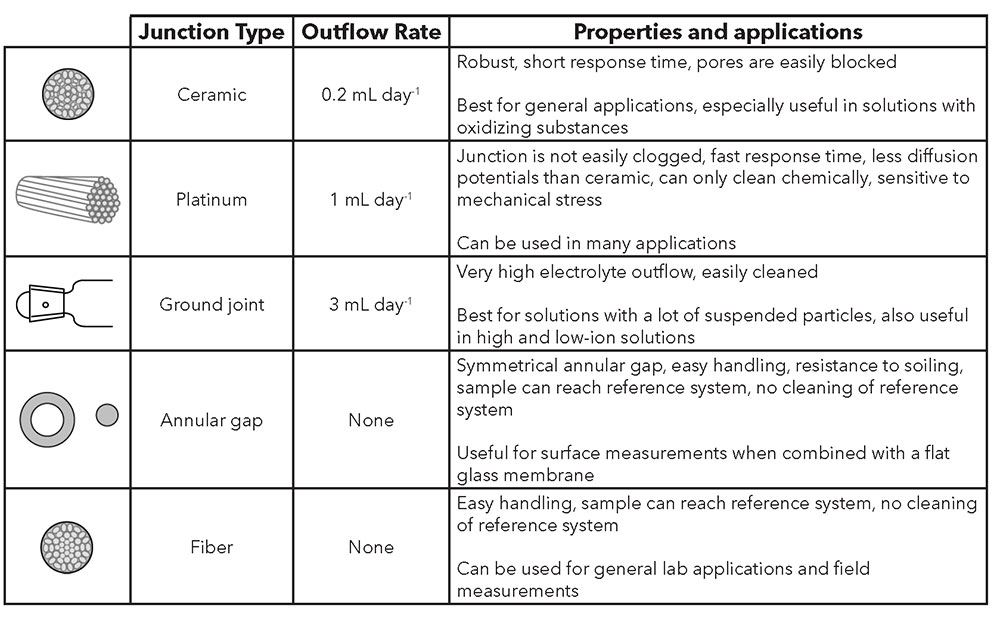
Ceramic
A ceramic junction uses the porosity of unglazed ceramic. Ceramic junctions have a KCl outflow rate of ~0.2 mL/day and a relatively high electrical resistance (1 kΩ). Diffusion potentials are easily created in measurement solutions with greater ionic strength, as the concentration gradient at the junction is very large. In lower ionic strength solutions, the resistance of the test material may be too high for exact measurements. Both effects are amplified by the low outflow rate, so ceramic junctions are less suitable in such cases. Due to the high risk of blockage of its pores, it is also not suitable for solutions containing suspended particles. Only in measurement solutions that contain oxidizing substances is the ceramic junction superior to the platinum junction.
Platinum
The platinum junction consists of fine, twisted platinum filaments between which the electrolyte flows out along precisely defined channels. The platinum junction features a very constant outflow and does not easily become blocked. With an outflow rate of ~ 1 mL / day and electrical resistance of ~ 0.5 kΩ, it has advantages over ceramic junctions. The platinum junction is more sensitive to mechanical stress and is also less than optimal for strongly oxidizing or reducing solutions due to the occurrence of disruptive potentials. However, the platinum junction can be used almost universally.

Figure 10: TruLine pH 17 electrode with platinum junction
Ground-Joint
The ground-joint junction works with the thin gap of the unlubricated ground glass as an outflow opening for the electrolyte. The outflow rate is ~ 3 mL / day and greater. It features a very low electrical resistance (0.1 kΩ). The ground-joint junction is suitable for measurements in contaminated solutions, as it is easy to clean. Due to the high outflow rate, it is suitable for both high and low-ion solutions.

Figure 11: Science pHT-G electrode with ground-joint junction
Plastic
Junctions constructed from plastic fibers can also be used for special applications. For example, combination electrodes with a plastic shaft often have junctions made from nylon fibers to avoid contamination of the connection hole. For process measurements in solutions that contain fluoride, electrolyte keys with PTFE junctions are used.

Figure 12: TruLine pH 25 electrode with plastic fiber junction
Additional Reference Junctions
Additional junctions can be used to prevent contamination of the reference system. With this design, the reference electrode is immersed in electrolyte solution within an additional chamber. This additional chamber acts as an extra barrier against contamination while additional junctions are used to ensure the reference system still has contact with the measurement solution. The reference system can still become contaminated by the measurement solution, but the solution must first diffuse through the additional junction(s).
The Silamid reference system by YSI is a special type of double junction electrode that utilizes a unique construction of the silver/silver chloride reference system. Most electrodes featuring a Ag/AgCl system are built with an Ag wire coated with AgCl. Silamid reference systems have a glass tube with the inner part coated with Ag, then filled with AgCl, and plugged with a polyester fiber. This reference system creates greater contact surface area between Ag and AgCl compared to the standard Ag/AgCl wire system, resulting in a reference system that is long lasting and very stable. The Science series of electrodes from YSI feature the Silamid reference system.
YSI offers a variety of platforms for the measurement of pH. Whether for the lab (MultiLab, TruLab), environmental sampling (ProQuatro, ProSwap, ProDSS, Pro10), or for long-term monitoring (EXO1, EXO2), YSI has what you need!

Additional Blog Posts of Interest
Why is the pH Scale Logarithmic?
Is pH the Measurement of Hydrogen Ion Concentration or Ion Activity?
pH Measurement Methods - Advantages and Disadvantages
pH Meter Calibration Problems? Check Out These 12 Tips!