Why is pH logarithmic?
The pH scale is logarithmic, essentially meaning the difference in 1 pH unit is a difference of 10 times! In a previous blog post, we introduced exactly what we are measuring when we take a pH measurement – hydrogen ion activity or concentration.
Importance of pH Measurement
pH is an incredibly important parameter that is measured in nearly every water quality application. It plays a role in the taste (acid = fresh, neutral = bland, and alkaline = inedible) and the preservation of food. In environmental sampling and monitoring, high or low pH values can be indicative of pollution. In wastewater treatment, pH is regulated as part of a National Pollutant Discharge Elimination System (NPDES) permit and many processes are pH-dependent. These are just some of the applications in which pH plays a critical role.
pH and the pH Scale
What does "pH" stand for? The term pH originates from Latin and is an acronym for "potentia hydrogenii" - the power of hydrogen. The pH scale is commonly used to represent hydrogen ion activity.
On the pH scale, pH values below 7 represent acidic solutions (hydrogen ion activity greater than hydroxide ion activity) while values above 7 represent basic solutions. At pH = 7, hydrogen ion and hydroxide ion activity are equal (Table 1).
As can be seen in Table 1, the possible range of hydrogen (H+) and hydroxide (OH-) ion activity can span many orders of magnitude. In order to easily manage and represent the wide range of ion activities, a logarithmic pH scale is used. Other parameters that have an incredibly wide range of possible values also use a logarithmic scale, such as the measurement of sound using the decibel (dB), or measurement of the energy released from an earthquake using the Richter scale.
Table 1: Hydrogen ion and hydroxide ion activities on the pH scale
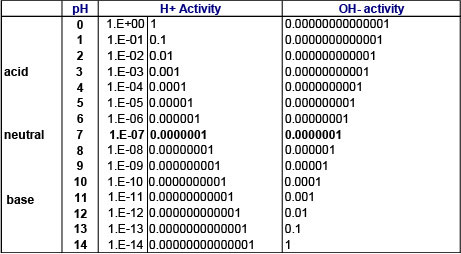
A change on the pH scale of 1.0 pH unit indicates that hydrogen ion activity differs by an order of magnitude (i.e. factor of 10). For example, hydrogen ion activity at pH 4 is 10 times greater than at pH 5.
Due to the logarithmic nature of the pH scale, it is incorrect to simply average pH values and report them. Instead, it is more appropriate to report the median pH value or provide the range of pH values observed.
Equation [1] represents the determination of pH from the negative log base 10 of hydrogen ion activity.
[1] pH = -lg aH+
Conventional pH Values
Because of the difference between concentration and activity, the measurement of pH cannot be directly based on the concentration of hydrogen ions in solutions. In addition, it is not possible to achieve an absolute calibration of the pH scale against the concentration of hydrogen ions. Such a calibration would always be an approximation only.
Therefore, in practice, the pH measurement is based on a conventional pH scale. Conventional pH values are measured in comparison to the pH values of standard buffer solutions. Provided that calibration and measurement are completed carefully, this makes all pH values comparable, regardless of the probe or measuring equipment used to record them.
We encounter items of varying pH every day, as can be seen in Figure 1 below.
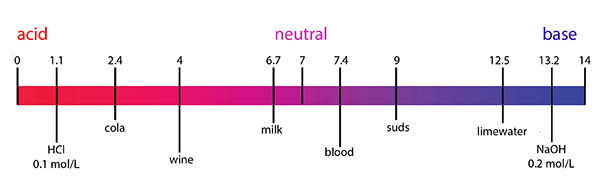
Figure 1: pH values of everyday items
YSI offers a variety of platforms for the measurement of pH. Whether for the lab (MultiLab, TruLab), environmental sampling (Pro Quatro, ProDSS, Pro10), or for long-term monitoring (EXO1, EXO2), we have pH measurement solutions!

Additional Blog Posts of Interest:
Is pH the Measurement of Hydrogen Ion Concentration or Ion Activity?
pH Measurement Methods - Advantages and Disadvantages
Extend the Life of Your pH Electrode in 3 Practical Steps
pH Meter Calibration Problems? Check Out These 16 Tips!