pH measurement is an important parameter in nearly every water quality application. In wastewater treatment, pH is regulated as part of discharge permitting and many treatment processes are pH dependent. In environmental sampling and monitoring, high or low pH values can be indicative of pollution.
Formation of the Hydrogen Ion
pH describes how acidic or basic a solution is. Whether an aqueous solution reacts as an acid or a base depends on its hydrogen ion (H+) content. In fact, the term "pH" originates from Latin and is an acronym for "potentia hydrogenii" - the power of hydrogen.
Even chemically pure, neutral water contains hydrogen ions due to the autodissociation of water [1]. In this process, water molecules are broken up into simpler constituents (i.e. ions).
[1] H2O ⇔ H+ + OH-
In this reaction, H2O is deprotonated (i.e. loses a proton). This results in the formation of a positively charged hydrogen ion (H+) and a negatively charged hydroxide ion (OH-). The hydrogen ion is customarily used to represent a proton.
The hydrogen ion does not remain a free proton for long, as it is quickly hydrated by a surrounding un-ionized water molecule. The formation of the resulting ion, the hydronium ion, is represented in equation [2]:
[2] H2O + H+ ⇔ H3O+
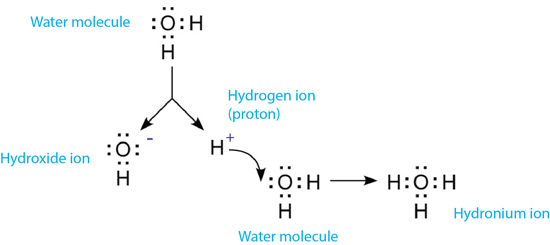
The dissociation of water and the formation of the hydronium ion.
At equilibrium conditions (750 mmHg and 25 °C), 1 L of pure, neutral water contains 10-7 mol H+ and 10-7 mol OH- ions.
Definition of Acid and Base
Acids are substances which release hydrogen ions (i.e. protons), so a solution is considered acidic if it contains more hydrogen ions than neutral water.
Bases are substances which accept hydrogen ions. When bases are dissolved in water, they bind to some of the hydrogen ions formed from the dissociation of the water. Basic solutions contain fewer hydrogen ions than neutral water.
Aqueous solutions are considered acidic if they contain more than 10-7mol/L of hydrogen ions and basic if they contain fewer than 10-7mol/L of hydrogen ions at 25 °C.
Acids and bases neutralize each other, resulting in the formation of water and salt. An example would be the reaction of sodium hydroxide (NaOH) and hydrochloric acid (HCl) in equation [3] below:
[3] NaOH + HCl ⇔ NaCl + H2O
A reaction between an acid and base involves a transfer of protons. In the above acid-base reaction, a proton is donated from HCl (acid) to NaOH (base) to form sodium chloride (NaCl) and water.

Sodium chloride in the form of sea salt.
Is pH the Measurement of Hydrogen Ion Concentration or Hydrogen Ion Activity?
Ions carry either a positive (e.g. H+) or negative (e.g. OH-) charge. As charge carriers, all dissolved ions exert electrical forces on their surroundings. While a solution might be electrically neutral on a macroscopic scale, the effects of ions can be drastic at the microscopic scale.
Solutions with a relatively high concentration of ions may yield a determination of ion concentration that is unusually low. Therefore, solutions begin to behave as if some of the ions are no longer present. This apparent loss of ions is caused by the interaction of ions in solution, ultimately resulting in significant deviations from ideal behavior. In order to take this interaction into account, the ion activity, also known as the effective ion concentration, must be considered rather than ion concentration. As a result, pH is a measure of hydrogen ion activity.
[Read Blog Post: Is pH the Measurement of Hydrogen Ion Concentration or Ion Activity?]
Why is the pH Scale Logarithmic?
The pH scale is commonly used to represent hydrogen ion activity. On the pH scale, pH values below 7 represent acidic solutions (hydrogen ion activity greater than hydroxide ion activity) while values above 7 represent basic solutions. At pH = 7, hydrogen ion and hydroxide ion activity are equal.
As can be seen in table 1, the possible range of hydrogen (H+) and hydroxide (OH-) ion activity can span many orders of magnitude. In order to easily manage and represent the wide range of ion activities, a logarithmic pH scale is used.
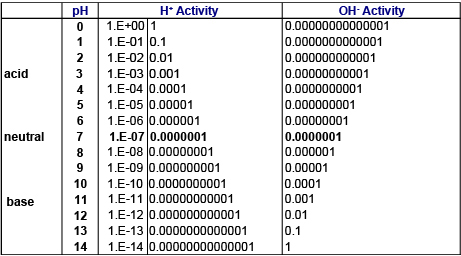
Hydrogen ion and hydroxide ion activities on the pH scale.
A change on the pH scale of 1.0 pH unit indicates that hydrogen ion activity differs by an order of magnitude (i.e. factor of 10). For example, hydrogen ion activity at pH 4 is 10 times greater than at pH 5.
Due to the logarithmic nature of the pH scale, it is incorrect to simply average pH values and report them. Instead, it is more appropriate to report the median pH value or provide the range of pH values observed.
Equation [4] represents the determination of pH from the negative log base 10 of hydrogen ion activity.
[4] pH = -lg aH+
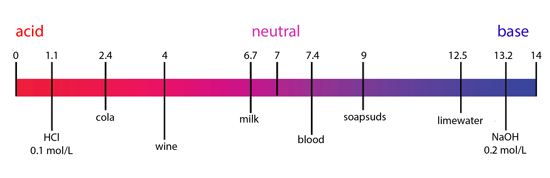 pH values of everyday items.
pH values of everyday items.
[Read Blog Post: Why is the pH Scale Logarithmic?]
pH Measurement Methods - pH Meter vs pH Strips
Visual, photometric, and potentiometric methods can be used to measure the hydrogen ion activity of a solution. Visual and photometric methods rely on color changes of specific organic pigments in order to determine pH. Visual methods are completed with visual indicators such as pH test strips, while photometric determination involves shining a light through the sample and measuring the absorbance.
The application of visual or photometric determination of pH is limited. Measurements will be unreliable if the solution to be measured is cloudy or has an inherent color. Some measurement solutions also contain chemical bonds which destroy the color indicators through oxidation or reduction and produce incorrect results.
 While pH test strips can be helpful, they are not as reliable as a pH electrode.
While pH test strips can be helpful, they are not as reliable as a pH electrode.
Potentiometric methods determine pH by using the electrical potential of pH-sensitive electrodes as a measurement signal, which is then displayed by a pH meter. The disadvantages of visual and photometric methods are not present with potentiometric methods, as potentiometric sensors are very sensitive, selective, and can be used in almost any application.
How Does a pH Electrode Work?
A distinction is made between hydrogen, metal (e.g. antimony), and glass potentiometric electrodes, with the glass pH electrode being the most commonly used pH sensor. For more details on the hydrogen and metal electrodes, check out the YSI pH Handbook!

The glass pH sensor is an example of an ion selective electrode (ISE). This system consists of the ISE reacting on a special ion type, in this case the hydrogen ion, and a reference electrode that are jointly immersed in the sample to be measured.

A pH electrode is technically a hydrogen ion selective electrode (ISE). The only major difference between a pH electrode and a nitrate ISE is the membrane used.
The hydrogen ISE provides an electrochemical potential (e.g. signal) that is influenced by the hydrogen ion activity of the solution. The reference electrode, however, maintains an electrochemical potential that does not depend on the composition of the sample. The difference between these potentials, the voltage (mV) displayed on a pH meter, determines the pH value based on the Nernst equation.
Importance of the Nernst Equation
The Nernst equation establishes a relationship between the measured voltage and the ion activity of the solution. The slope of the electrode for a change in one pH unit can be described by a portion of the Nernst equation called the Nernst slope (S).
[5] S = -2.303 RT/nF
The variables R and F are constants and therefore not of further concern. Since the electrode slope (i.e. electrode response) is dependent upon the temperature (T) of the solution, it is very important that pH measurements be completed with an accurate measurement of temperature. The theoretical Nernst slope at 25 oC is -59.16 mV. The n variable signifies the charge on the ion, which is +1 for the hydrogen ion (H+).

pH Electrode Design
The main components of a glass pH electrode are the electrode body, pH-sensitive glass membrane, reference electrode (i.e. reference system), reference electrolyte, and the reference junction.
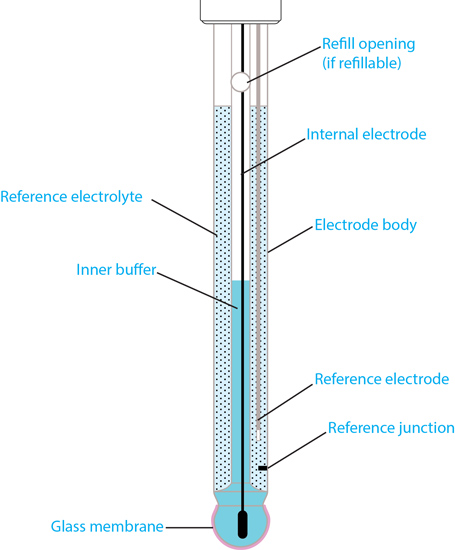 Structure of a typical combination pH electrode with glass body.
Structure of a typical combination pH electrode with glass body.
pH Electrode Body
The term ‘glass electrode’ is not indicative of the material used to construct the electrode body, as electrodes can have either a plastic or glass electrode body. Rather, ‘glass electrode’ is used to describe the membrane material (i.e. glass membrane).
Plastic body electrodes are more rugged and less likely to crack than glass, while glass electrodes typically possess a greater range of operating temperature. Glass electrodes are also commonly refillable.
 The YSI ProDSS handheld instrument features plastic pH and pH/ORP sensing modules that are replaceable, but the rest of the sensor body is titanium to provide extra protection in the field.
The YSI ProDSS handheld instrument features plastic pH and pH/ORP sensing modules that are replaceable, but the rest of the sensor body is titanium to provide extra protection in the field.
pH Glass Membrane
With the glass pH electrode, a glass membrane is fused on as a pH sensor. This membrane is filled with a buffer solution of known pH (typically pH = 7). This electrode design creates an environment with constant binding of H+ ions on the inside of the glass membrane, while the outside of the glass membrane is exposed to the sample where a variable amount of H+ ions exist. The difference in H+ ions creates a potential that is read versus the stable potential of the reference electrode.
In order to ensure optimal moistening of the glass membrane, the membrane shape can vary. Sphere and cone membrane shapes can be used for most applications, but unique applications may require a specialized membrane, such as the spear tipped membrane for penetrating semi-solid media and a flat membrane for surface measurements.

YSI TruLine 21 (spear tip membrane) and YSI TruLine 27 (flat membrane).
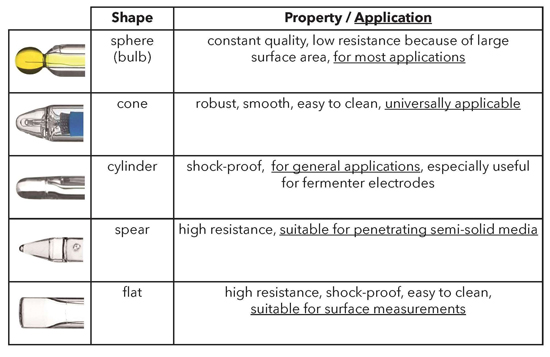 Shapes and properties of glass pH membranes.
Shapes and properties of glass pH membranes.
Types of Reference Electrodes
The reference electrode/system and the hydrogen ISE (i.e. the electrode with the glass membrane) can be separate electrodes or they can be combined into a single electrode for convenience. The combination-style electrode is very common.
Regardless of the electrode design or the type of reference system used, the reference electrode is immersed in reference electrolyte (typically KCl).
The most common type of reference electrode used today is the silver/silver chloride (Ag/AgCl) system. Since silver is non-toxic to humans, Ag/AgCl electrodes can also be used in medicine and food technology where the poisonous mercury and thallium systems are prohibited. Disposal is also less critical with Ag/AgCl than with thallium and mercury. Ag/AgCl has a wide range of application with respect to temperature (up to 140 °C) and is therefore also suitable for sterilizable electrodes. Most electrodes feature a Ag/AgCl reference system.
The iodine/iodide system, a relatively new reference system with a fast response time, has recently been developed. Compared to conventional electrodes with Ag/AgCl reference systems, electrodes with iodine/iodide reference systems have the advantage of much lower temperature sensitivity, as the temperature coefficient of this reference system is almost zero.
The iodine/iodide system is also metal ion free, a feature that is especially useful when measuring in Tris buffer and protein solutions. Reference systems with metal ions (e.g. Ag/AgCl) will interact with these solutions, ultimately resulting in the reference junction becoming clogged.
[Read Blog Post: Anatomy of pH Electrodes]
Reference Electrolyte Function and Qualities
The reference electrolyte has a connection to the sample through the junction, as it serves to close the electrical circuit in the electrode.
A good reference electrolyte must have certain qualities. The reference electrolyte must have good electrical conductivity and be chemically neutral. Since some electrolyte will typically leak into the sample during measurement, it is also important the electrolyte not react with the measurement solution.
The ions of an electrolyte solution must also be equally mobile. If ions in the electrolyte solution diffuse at different rates, an electrical potential (diffusion potential) can form due to the division between the positive and negative charge. This unwanted potential can be problematic when measuring pH. With some ions (e.g. K+ and Cl-), the differences in diffusion speed are small, resulting in a much smaller diffusion potential.
Potassium chloride (KCl) possesses all of these qualities. As a result, KCl is the most commonly used electrolyte solution.
Liquid, Gel, and Polymer Electrolyte
Electrodes can have a gel, polymer, or liquid electrolyte. pH electrodes with liquid electrolyte can typically be refilled, resulting in a longer electrode life. Unlike an electrode with gel electrolyte, liquid electrolyte can easily be drained and replaced if it becomes contaminated.
If using a refillable electrode (e.g. the TruLine pH 15), it is important to remember the refilling opening must always be open during calibration and measurement! Also, ensure the fill level of the electrolyte is at least 2 cm above the level of the calibration and/or measurement solution.
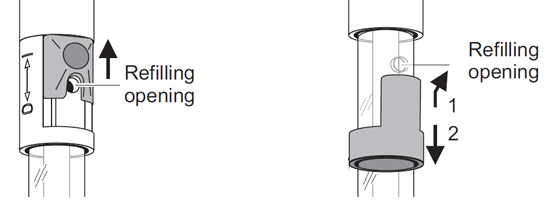 The refill opening on refillable lab electrodes must always be open when measuring or calibrating.
The refill opening on refillable lab electrodes must always be open when measuring or calibrating.
Response time is typically faster with refillable electrodes. Also, electrodes with liquid electrolyte have fewer limitations in their range of use, as gel and polymer electrolytes have a lower resistance to temperature and temperature changes. Unlike electrodes with liquid electrolyte, the incredibly small outflow rate of gel and polymer electrolyte in strongly acidic, basic, and low-ionic strength solutions can result in measurement errors due to the formation of diffusion potentials.
Gel electrolyte still consists of KCl, but a gelling agent is added in order to prevent the electrolyte from readily leaking into the sample via the reference junction during measurement. Since there is virtually no loss of the electrolyte, these electrodes are easier to maintain, as there is no need to refill them with electrolyte. Since they cannot be refilled, electrodes with a gel electrolyte have a shorter lifetime than those with a liquid electrolyte.
 The YSI TruLine pH 25 electrode has gel electrolyte and a plastic body.
The YSI TruLine pH 25 electrode has gel electrolyte and a plastic body.
Polymer electrolyte is solid and can directly contact the sample during measurement. Due to the absence of electrolyte outflow, the mobility of all ions is greatly limited. This results in no precipitation of silver at the junction and makes the diffusion of foreign ions into the electrode virtually impossible.
pH Reference Junction
The reference junction, also known as a diaphragm, creates electrical contact between the reference system and the solution. Much like the reference electrolyte, the reference junction must possess certain qualities.
Diffusion voltages at the junction are a common measurement error, so the junction plays a major role in the precision of measurements. To keep these disruptive potentials small, the junction must guarantee a relatively large and consistent outflow of reference electrolyte. However, the junction must only be slightly permeable to prevent electrolyte from escaping too quickly, which is especially important with electrodes utilizing liquid electrolyte. Different junction types have different outflow rates of electrolyte.
In addition to the permeability of the junction, its electrical resistance should be as low as possible and it must be chemically inert.
Types of Reference Junctions
There are several types of junctions, each with unique characteristics.
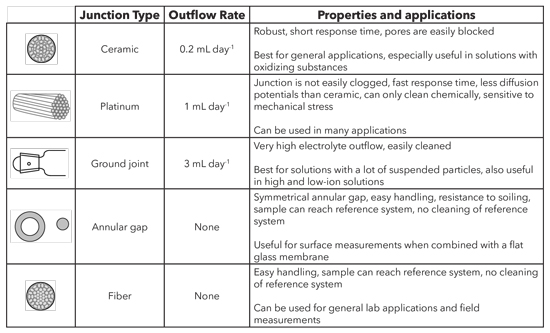 Types of reference junctions.
Types of reference junctions.
Ceramic
A ceramic junction uses the porosity of unglazed ceramic. Ceramic junctions have a KCl outflow rate of ~0.2 mL / day and a relatively high electrical resistance (1 kΩ). Diffusion potentials are easily created in measurement solutions with greater ionic strength, as the concentration gradient at the junction is very large. In lower ionic strength solutions, the resistance of the test material may be too high for exact measurements. Both effects are amplified by the low outflow rate, so ceramic junctions are less suitable in such cases. Due to the high risk of blockage of its pores, it is also not suitable for solutions containing suspended particles.
Platinum
The platinum junction consists of fine, twisted platinum filaments between which the electrolyte flows out along precisely defined channels. The platinum junction features a very constant outflow and does not easily become blocked. With an outflow rate of ~ 1 mL / day and electrical resistance of ~ 0.5 kΩ, it has advantages over ceramic junctions. The platinum junction is more sensitive to mechanical stress and is also less than optimal for strongly oxidizing or reducing solutions due to the occurrence of disruptive potentials. However, the platinum junction can be used almost universally.
 The YSI TruLine pH 17 electrode features a platinum junction.
The YSI TruLine pH 17 electrode features a platinum junction.
Ground-joint
The ground-joint junction works with the thin gap of unlubricated ground glass as an outflow opening for the electrolyte. The outflow rate is ~ 3 mL / day and greater. It features a very low electrical resistance (0.1 kΩ). The ground-joint junction is suitable for measurements in contaminated solutions, as it is easy to clean. Due to the high outflow rate, it is suitable for both high and low-ion solutions. The Science pHT-G is what we typically recommend when there are a lot of suspended particles in the sample.
 The YSI Science pHT-G electrode features a ground-joint function with fast electrolyte outflow. This outflow rate keeps the junction clean and provides fast measurement results.
The YSI Science pHT-G electrode features a ground-joint function with fast electrolyte outflow. This outflow rate keeps the junction clean and provides fast measurement results.
Additional Reference Junctions
Additional junctions can be used to prevent contamination of the reference system. With this design, the reference electrode is immersed in electrolyte solution within an additional chamber. This additional chamber acts as an extra barrier against contamination while additional junctions are used to ensure the reference system still has contact with the measurement solution. The reference system can still become contaminated by the measurement solution, but the solution must first diffuse through the additional junction(s).
[Read Blog Post: Anatomy of pH Electrodes to learn more about reference junctions]
Selecting the Right Electrode
There is not a pH electrode currently available that can be used for every possible application, as there are different requirements for different applications.
There are many different applications, especially in the lab! Unique applications may require a specific shaft material, reference system, junction type, number of junctions, reference electrolyte, and/or membrane shape. Due to the wide range of lab pH electrodes, we encourage you to check out the
YSI pH Electrode Selection Guide and the
YSI pH Electrode Application Guide when searching for your next lab electrode!
Ask a Question
Importance of Temperature
A variable in the Nernst equation is temperature, so the response (i.e. slope) of a pH electrode is dependent upon temperature. Therefore, pH measurements should be completed with an accurate measurement of temperature.
The pH values of buffer solutions are temperature dependent and the response can vary from manufacturer to manufacturer. As a general rule, basic buffer solutions exhibit stronger temperature effects than acidic ones. Modern pH meters automatically adjust for the respective temperature profile once the buffer set being used has been correctly set.
 YSI always recommends having an accurate measure of temperature when taking pH measurements. Some pH electrodes have a built-in temperature sensor, while external temperature sensors are also available (e.g. ScienceLine Temp 135 and ScienceLine Temp 136) if the pH electrode doesn't have a built-in temperature sensor.
YSI always recommends having an accurate measure of temperature when taking pH measurements. Some pH electrodes have a built-in temperature sensor, while external temperature sensors are also available (e.g. ScienceLine Temp 135 and ScienceLine Temp 136) if the pH electrode doesn't have a built-in temperature sensor.
pH Buffer Solutions
Buffers are aqueous solutions whose pH remains virtually unaltered by the addition of small quantities of acids or bases. Buffer solutions are capable of binding hydrogen ions with the addition of acids and releasing hydrogen ions with the addition of bases.
Buffer solutions are often colored to clearly differentiate them from one another during calibration.
The composition of buffer solutions varies depending on the manufacturer. When selecting a buffer set, care must be taken to ensure they are made according to the formula established by the National Institute of Standards and Technology (NIST). These buffers have pH values of 4.01, 6.86, and 9.18. Alternatively, NIST traceable buffers are also sufficient for use when calibrating. YSI buffers are NIST traceable and are offered in values of pH 4, 7, and 10.
Check out the YSI pH Electrode Calibration Guide for some practical tips on calibration!
What Does Calibration Do?
pH calibration does two things – 1.) establish a new electrode slope based on the previously discussed Nernst equation and 2.) set the zero point. Since both of these can change over time, frequent calibration is necessary.
The zero point, also known as the asymmetry potential/point, is typically the mV value when the electrode is placed in pH 7 buffer. The theoretical zero point is, not surprisingly, 0 mV. This is true because the reference electrode is typically in a solution of electrolyte that has a pH of 7. If the reference and the sensing electrode are both in a solution with the same pH, there should theoretically be no difference in their potentials, resulting in a display of 0 mV on the pH meter. A new electrode will have an asymmetry potential that is typically only a few mV if it has been carefully prepared.
The zero point is helpful when determining the operating condition of the electrode. If the asymmetry point begins to drift too far from zero, the electrode may need to be cleaned, serviced, or replaced. The asymmetry point will change as the electrode ages, so regular calibration is recommended and will be required more frequently as the electrode ages to compensate for these changes.
How Often Should I Calibrate?
Perhaps the most common question regarding calibration is how often it should be completed. The frequency of subsequent calibrations depends on the application. Some applications require daily calibration while others may require only weekly or monthly calibration. If possible, calibration at the beginning of each day is best.
[Read Blog Post: pH Measurement Calibration Problems? Check Out These 12 Tips]
More frequent calibration is necessary when measuring in heavily contaminated or low-ion samples. pH electrodes used in strongly acidic and high temperature environments also need to be calibrated more often, as these electrodes will age much faster, resulting in a slower response, change in electrode slope, and drifting of the zero point.
How Many Calibration Points Should I Use?
The number of calibration points is a common topic of discussion. Most lab and field instruments will allow for calibration with up to 3 buffers.
Calibration may be finished after one buffer, resulting in the completion of a single-point calibration. The zero point is determined during a single-point calibration while the electrode slope used is the theoretical Nernst slope (-59.16 mV/pH at 25 °C). It should be noted that a single-point calibration must be completed with pH 7 buffer.
The range of use of single-point calibration is limited, as an electrode calibrated to one point (i.e. pH 7) should only be used to measure within a range of 6.5 pH to 7.5 pH. The pH value obtained may be used to compare to previously obtained measurement results, but is not an absolute value.
It is best to perform at least a two-point calibration, using pH 7 buffer as one of those points (6.86 for NIST buffer set). Although it is not required for many instruments, it is best to start with pH 7 buffer. For a two-point calibration, the pH buffers used should differ by at least two pH units and should bracket the expected in situ pH conditions. Unless the sample is expected to be above pH 7, basic buffers should not be used, as their pH value changes by absorbing CO2.
Three-point calibrations are typically completed when the sample pH conditions are not well understood. Asymmetry and slope are determined for both two and three-point calibrations.
What is Electrode Efficiency?
After calibration, pH meters will typically display the slope of the electrode in either mV/pH (i.e. the Nernst slope), as a percentage, or both. We are often asked what this percentage, termed the electrode efficiency, actually means.
Electrode efficiency is simply a percentage of the theoretical Nernst slope (-59.16 mV/pH).
[6] Electrode Efficiency = (Observed Slope / Theoretical Slope) * 100
As an example, if the slope was determined to be -57.10 mV/pH during calibration, the efficiency would be 96.52%.
Interested in learning more about pH? Check out the following resources!

Helpful Links - Handbooks and Guides
The YSI pH Handbook
Lab pH Electrode Selection Guide
Lab pH Electrode Application Guide
Lab and Field pH Electrode Calibration Guide
