Besides runoff from croplands and lawns, sources of nitrate in the environment include discharge from wastewater treatment plants, septic systems, manure storage areas, and industrial facilities.1 Natural sources of nitrate, such as decaying plant debris, also exist.
Nitrate is measured in many applications – surface water, groundwater, wastewater, aquaculture, food, and more – but the reasons for monitoring nitrate vary.
Nitrate in Surface Water
When excess nutrients – such as nitrate and phosphorus – wash into a body of water, harmful algal blooms (HABs) are often the result. These blooms can produce dangerous toxins and cause a drop in dissolved oxygen, resulting in fish kills. Algal blooms in water bodies that serve as drinking water sources are especially problematic.
Because of the dangers of HABs, many water quality monitoring programs aim to determine when excess levels of nitrate are present. Check out our blog post, HABs | Everything You Need to Know, to learn more.

A HAB in Lake Erie caused by agricultural runoff. Lake Erie serves as the source of drinking water for cities like Toledo, OH. Photo: Alliance for the Great Lakes
Nitrate in Drinking Water
Nitrate can cause other health issues when consumed, especially in young children. Exposure to drinking water with high nitrate levels can result in methemoglobinemia, also known as "blue baby syndrome." This condition often occurs when water with excessive nitrate is used to prepare infant formula.
Manufacturers of ready-to-feed infant formula can use various methods to measure nitrate in their product, such as the method available for our FS 3700 Automated Chemistry Analyzer.
Nitrate in drinking water can cause health issues, primarily when it is used to prepare infant formula.
When babies ingest nitrate, it is reduced to nitrite by bacteria in the mouth and stomach – an infant's stomach is less acidic than adults. Nitrite binds to hemoglobin to form methemoglobin, a special type of hemoglobin that cannot release oxygen to cells.2
The most common symptom of "blue baby syndrome" is a gray-blue color that develops on a child's lips; this can eventually spread to the rest of the body, resulting in death in the most severe cases.3
Adults can also develop methemoglobinemia and experience elevated heart rate, weakness, nausea, and even death.3
Nitrate in Wastewater
Besides nitrate, other chemical forms of nitrogen, such as nitrite and ammonia, can be found in the environment. The nitrogen cycle describes how these transform from one form to another. Want to learn more about the nitrogen cycle? Check out this nitrogen cycle overview from the University of Minnesota.
One of the goals of wastewater treatment is to remove nitrogen to prevent over-enrichment of receiving waters. Processes in the nitrogen cycle – specifically nitrification and denitrification – play a vital role in accomplishing this goal.

Nitrate monitoring is critical for process control of wastewater treatment. An IQ SensorNet NitraVis sensor is shown in an aeration basin.
Ammonia is converted to nitrite and ultimately nitrate through a process called nitrification. This process occurs with the help of aerobic bacteria – these need oxygen to survive.
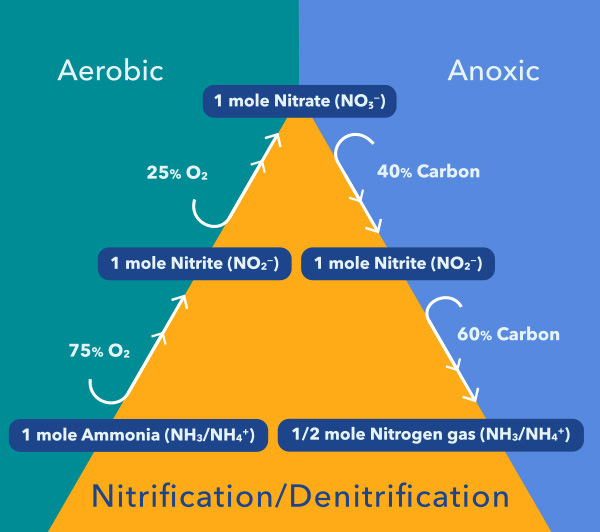
Two phases of the nitrogen cycle – nitrification and denitrification – are particularly important in the treatment of wastewater. This pathway diagram shows how these phases work together to transform ammonia into nitrogen gas.
Nitrite and nitrate, collectively termed (NOx), are converted to nitrogen gas and removed from wastewater by a biological reaction known as denitrification. This process requires very low dissolved oxygen (DO) and a sufficient supply of organic carbon, so nitrate and COD/BOD/DOC monitoring are critical for denitrification process control.
Nitrate in Aquaculture
As discussed earlier, high levels of nitrate can fuel HABs, resulting in fish kills. But does nitrate in water directly impact aquatic species when no HAB is present?
Natural nitrate levels are relatively low – 1 mg/L or less – in some surface waters.1 At this level, nitrate is not harmful, but adverse health effects can be seen in aquatic species when nitrate concentration gets too high.
One study conducted by the Conservation Fund’s Freshwater Institute investigated the effects of chronic exposure of aquaculture-raised rainbow trout to modest levels of nitrate-nitrogen (75 to 100 mg/L). Results suggested deformities and significant behavioral changes in recirculating aquaculture systems (RAS) at these levels. Check out our blog post on this topic, Nitrate Levels in Aquaculture May be More Dangerous Than You Think.

A recirculating aquaculture system (RAS) in Thailand.
Nitrate levels can build up in a RAS as fish excrete ammonia and biofiltration systems convert it to nitrate. Monitoring for nitrate can help inform aquaculture managers when to adjust operations – this could include increasing the rate of water exchange, adding a denitrification unit, or reducing feed load.
Nitrate in Food
Nitrate is commonly used as a preservative for bacon, ham, salami, and some cheeses. Studies have tied the use of nitrate to the formation of nitrosamines, some of which are cancer-causing compounds. One way nitrosamines can form is when nitrites – naturally converted from nitrate in the body – are located near proteins; this condition is found in protein-rich foods such as preserved meats. When preserved meats are cooked at high temperatures, it is easier for nitrosamines to form.4

Grilling preserved meats can result in the formation of nitrosamines, some of which are known to cause cancer.
While nitrate’s use as a preservative receives a considerable amount of attention, humans receive very little of their dietary nitrate intake from processed meat – only about 5%. Most of the dietary nitrate (>80%) we receive is from vegetables, with leafy green vegetables often accumulating high concentrations of nitrate.4
Rather than being added as a preservative, vegetables acquire nitrate from the soil in which they grow, with those grown in soil enriched with fertilizer having higher levels than organic produce.5 Nitrates in vegetables are less likely to form nitrosamines, as they aren’t protein-rich foods. Veggies also contain compounds (e.g., vitamin C and polyphenols) that help minimize the chance of nitrosamine formation.4

Leafy green vegetables, like spinach, can contain high levels of nitrate. Compared to processed meats, nitrates in vegetables are less likely to form nitrosamines.
Nitrate in vegetables can be beneficial, as nitrate is tied to nitric oxide production.4 This gas may lower blood pressure and help fight infection, among other benefits.6 One thing to note – while it is possible to get so much nitrate from vegetables that methemoglobinemia can result, it is very unlikely to happen.4
Besides the impact of nitrate on the human body, another reason to monitor nitrate levels in vegetables is to optimize fertilization and harvest timing, as the concentration of nitrate in leafy greens can fluctuate throughout the day.7
So, how are nitrate levels measured in food? Some relatively inexpensive methods we cover in the next section – specifically colorimetric and potentiometric methods – can be used for nitrate analysis as long as an aqueous solution is created. One procedure for preparing a sample for analysis is to mix oven-dried leaf samples with aluminum sulfate extracting solution.7
Nitrate Measurement Methods
There are several different methods available when measuring nitrate in aqueous samples.
Colormetric
Colorimetric determination of nitrate involves the use of cadmium to reduce nitrate to nitrite. Nitrite is then reacted with another reagent to form a reddish dye. The intensity of the color produced is proportional to the nitrate concentration in the sample. YSI offers a few different instruments that measure nitrate in this way.
With direct-reading filter photometers, such as the 9800 Photometer, light is passed through a test tube containing the sample solution and then through a colored filter onto a photodetector. These instruments are relatively inexpensive and portable, but upwards of 15 minutes are required for reagents to thoroughly mix with each sample. Upon completion of the test, the mixture must be disposed of according to local regulations, which may take additional time and effort.

The 9800 Photometer measures nitrate in a variety of applications.
Our Flow Solution FS3700 Automated Chemistry Analyzer can be used to measure just about any aqueous sample (e.g., liquid milk and infant formula) in addition to cheeses (hard, semi-hard, soft, and processed), milk powder, and whey powder.
The FS3700 features flow injection analysis (FIA) technology, allowing for up to 30 samples to be processed per hour. The reaction takes place in the instrument, so there’s no need to manually mix reagent(s) and sample. The method detection limit is also quite low (0.016 mg/L), and measurements taken can be used for compliance reporting.

The FS3700 can be used to measure nitrate in a variety of samples – aqueous solutions, cheeses, milk powder, and more! The instrument can also process up to 30 samples per hour.
For more information on measuring nitrate with the FS3700, check out our methods document on Determination of Nitrate and Nitrite in Milk and Milk Products Using Cadmium Reduction and FIA with In-line Dialysis.
There are some drawbacks to instruments like the FS3700 – they are expensive, not portable, and it can take some technical expertise to get them up and running.
Potentiometric: Ion-Selective Electrode
Like other ion-selective electrodes (ISEs), the nitrate ISE sensor technically consists of two different electrodes:
- A sensing half-cell (i.e., the nitrate ISE) consists of a silver/silver chloride wire electrode that’s placed in a fill solution. This internal solution is separated from the sample by a polymer membrane that selectively interacts with nitrate ions.
- A reference electrode that does not react to the presence of nitrate.
The nitrate ISE provides an electrochemical potential that is influenced by nitrate in the sample. The reference electrode, however, maintains a constant potential. The difference between these potentials is the raw mV value that can be displayed on some instruments. This mV value is correlated to ion concentration – or ion activity – by the Nernst equation.
The Nernst Equation applies to the measurement of any ion with an ISE – nitrate, pH, chloride, and more. For more information on the Nernst Equation, check out the YSI pH Handbook.
Unlike the colorimetric method, the use of a nitrate ISE requires no reagent – no mixing required! It also only takes about one minute to obtain a stable measurement. These convenient features help explain why many choose to use an ISE.
Nitrate ISEs are not without their limitations, however. All nitrate ISEs require frequent calibration, as the sensor’s response to the nitrate ion changes over time. If the sensor will be used every day and accurate readings are needed – such as the use of our laboratory TruLine Nitrate ISE for compliance reporting of wastewater or drinking water samples – we even recommend calibrating the sensor at the beginning of each day. Check out our TruLine Nitrate ISE Calibration and Measurement Guide for more info on getting a lab ISE up and running.

YSI offers nitrate ISEs for wastewater (IQSN NitraLyt ISE; left), freshwater (ProDSS Nitrate ISE; Center), and lab applications (Truline Nitrate ISE; right).
Many YSI field instruments can be equipped with a nitrate ISE – ProQuatro, ProDSS, and EXO are some popular options. Unlike the TruLine Nitrate ISE, the ISEs for these platforms are designed to withstand harsh conditions in the field.
We also offer the NitraLyt ISE for the IQ SensorNet wastewater treatment process monitoring and control system. Simply put, this is the most rugged nitrate ISE we offer, primarily because long-term monitoring in a wastewater facility is one of the most challenging environments in which to measure water quality.
Check out our white paper on Best Practices for Wastewater Process Monitoring of Ammonium and Nitrate with Ion-Selective Electrode (ISE) Sensors for more on the design of a nitrate ISE and why nitrate monitoring is so important.
Spectrophotometric
Nitrate can be detected by measuring the penetration of ultraviolet (UV) light through a solution. This is accomplished using the principles of spectrophotometry.
As can be seen on the electromagnetic spectrum, forms of electromagnetic radiation (EMR) are distinguished by wavelength. Materials absorb EMR (light) differently, with nitrate absorbing most strongly at short ( < 250 nm) UV wavelengths. UV nitrate sensors measure this absorbance of light by nitrate and convert it to a concentration.
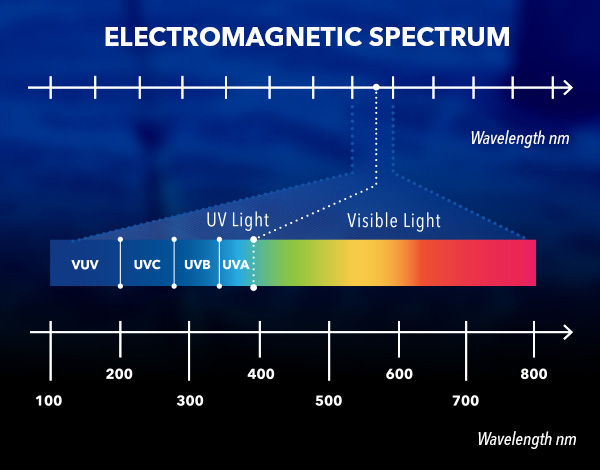
The earth is bathed in electromagnetic radiation (EMR), a form of energy. The forms of EMR are distinguished by wavelength, as described by the electromagnetic spectrum shown here.
UV nitrate sensors have some distinct advantages compared to colorimetric and potentiometric measurement methods. Like ISEs, UV nitrate sensors have a relatively fast response time and are reagentless – no chemicals, no mixing, no mess! They are more accurate than ISEs and require less frequent calibration.
There are some drawbacks to UV nitrate sensors, such as their size, high power requirement, and cost. These issues have been largely alleviated with the recent development of UV-LED technology – see the discussion on light sources below.

YSI offers UV nitrate sensors for wastewater (IQSN NitraVis; left) and freshwater (EXO NitraLED; right) applications.
All UV nitrate sensors have a few key components – a light source, sample path/gap, and a photodetector. There's no UV nitrate sensor available that can be used in every possible water quality application. The design of these key components typically determines suitable application(s) for the sensor. Sensor connections, processors, housing material, and other features can also vary.
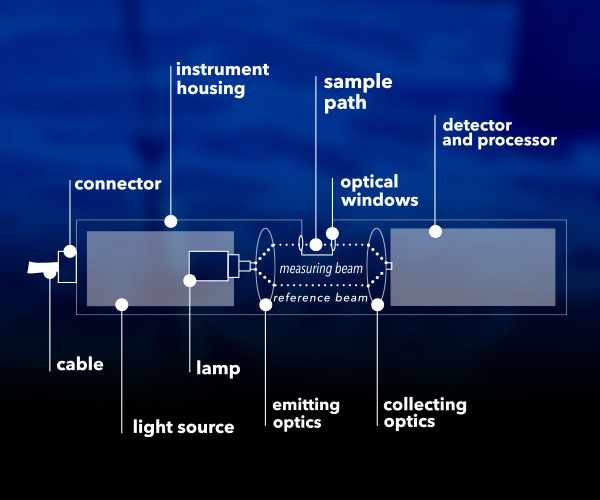
General design and key components of UV nitrate sensors.8
For more information on the design of UV spectrophotometric probes, check out our white paper on UV Vis Spectrophotometric Sensors for Wastewater Treatment Process Monitoring.
YSI offers two UV nitrate sensors – the YSI EXO NitraLED is designed for freshwater applications, while the IQ SensorNet NitraVis is specifically designed for wastewater treatment process and control. These nitrate sensors differ in the key components used.
Light Sources
Available light sources for UV nitrate sensors have different stability, output, brightness, lifetime, and power requirements.8 Sensors that feature xenon, mercury, or deuterium lamps typically require a lot of power, are bulky and are often times very expensive.
Recent advances in UV-LED technology have made possible much smaller UV sources that overcome the shortcomings of traditional lamps. The best example of this novel LED-based design is the YSI EXO NitraLED, as it is the same size as other EXO sensors, allowing it to be added to the payload of an EXO sonde. Compared to other UV nitrate sensors, the NitraLED is smaller, requires less power, and is the most affordable option for continuous, in situ monitoring in environmental applications.
Sensors with a lamp – such as the NitraVis – do have a performance advantage compared to current UV-LED technology. The peak of nitrate absorption is around 220 nm. The NitraVis is able to access this strong nitrate absorbance, whereas the NitraLED accesses nitrate absorbance at 235 nm, meaning it is less sensitive.
Optical Gap
The path/gap is the distance between the light source and the photodetector. When the sensor is immersed, the gap fills with solution. The gap is a critical component of sensor design, as absorption by nitrate increases linearly with an increase in the gap. It must be small enough for a sufficient amount of light to reach the photodetector but large enough for the amount of light absorbed by nitrate to be measured.
The application typically determines the gap length. In general, larger gaps are best for freshwater and coastal environments. Smaller gaps are best when there are high nitrate concentrations, high dissolved organic carbon (DOC) concentrations, or high total suspended solids (TSS) – these conditions are common at wastewater treatment plants. This explains why the EXO NitraLED has a 10 mm gap, while the IQ SensorNet NitraVis is offered with either a 1 or 5 mm gap.
Photodetectors
Photodetectors determine the amount of light that’s been attenuated as it crosses the gap and through the sample. Photodiodes or diode arrays are typically used, as they are more sensitive and stable than other types of detectors.8
While both the NitraLED and NitraVis utilize photodiode(s) as the photodetector, the NitraVis uses a diffraction grating that breaks up the light spectrum into 256 different ‘bins’ (i.e., channels) that can be independently looked at. The software algorithm then utilizes only a few channels, out of the 256 available, to correlate to nitrate while correcting for natural organic matter (NOM) and turbidity. These are important corrections, as NOM and turbidity can interfere with the nitrate measurement.
In contrast, the NitraLED filters only allow wavelengths of 235 nm (for nitrate) and 275 nm (for NOM) to pass through. An EXO turbidity sensor is then used for turbidity corrections – this is why a turbidity sensor is needed when using the NitraLED!
In summary, the detectors on these nitrate sensors are different, but they ultimately get to the same place – UV nitrate measurements that are compensated for NOM and turbidity.
Selecting the Right Nitrate Sensor
The first place to start when selecting a nitrate sensor is the intended application. The table below summarizes the ideal applications in which each of our nitrate measurement systems can be used.
There are plenty of other factors to consider besides application when selecting a nitrate sensor. A major consideration is the frequency of data collection. Discrete sampling datasets reflect 'snapshots' of nitrate data. These data are collected with portable instruments – ProQuatro and ProDSS – or from the analysis of grab samples in a lab with sensors like the TruLine Nitrate ISE or instruments like the FS3700.
In contrast, long-term monitoring platforms like the YSI EXO can be deployed for long periods, sometimes up to three months. These instruments are continuously collecting real-time data. Because high-frequency data is collected, no 'event' that changes water quality conditions is missed! With a more comprehensive understanding of water conditions, more informed decisions can be made.
Continuous monitoring results in the collection of high-frequency data that provide a much better idea of when water quality changes occur.
Another important consideration – for some, the only one – is the cost of a nitrate measuring system. Filter photometers and ISE-based lab and portable field systems have a similar acquisition cost, although digital field systems (e.g., ProDSS) are more expensive than analog systems (e.g., ProQuatro).
The EXO NitraLED is the most affordable UV nitrate sensor in the world for freshwater applications. This game-changing smart sensor integrates seamlessly into YSI's EXO platform—and its price point allows organizations to easily upgrade their water quality monitoring sites to collect continuous data with an incredibly stable nitrate sensor. This system is, by far, the best option for environmental monitoring of nitrate.
The IQ SensorNet system is the best option for those needing to monitor nitrate in wastewater, offering both UV and ISE sensor options. The NitraLyt ISE sensor provides exceptionally accurate and reliable ISE performance at a more affordable price. The NitraVis UV nitrate sensor represents the top tier of cost, but for good reason. These wastewater sensors are built into rugged, corrosion-resistant probes that are designed to measure accurately in incredibly harsh applications. They also feature UltraCleanTM ultrasonic technology to prevent fouling and lower maintenance requirements. IQ SensorNet nitrate sensors collect continuous data, allowing operators to monitor their process in real-time and react accordingly.
The Flow SolutionTM FS3700 Automated Chemistry Analyzer is the most expensive option offered for laboratory measurement of nitrate. The FS3700 can measure a variety of sample types quickly, efficiently, and with high accuracy. It is also ideal when analyzing samples for the purposes of compliance reporting.
Still not sure which nitrate sensing technology is right for your needs? Ask our experts or schedule a free virtual consultation today!
Sources
- EPA, Water: Monitoring & Assessment | Nitrates
- US National Library of Medicine National Institutes of Health, Drinking Water Nitrate and Human Health: An Updated Review
- Minnesota Department of Health, Nitrate and Methemoglobinemia
- British Broadcasting Corporation (BBC), The truth about the nitrates in your food
- Food Science & Nutrition, Effects of agriculture production systems on nitrate and nitrite accumulation on baby-leaf salads
- The Nobel Foundation, The Nobel Prize in Physiology or Medicine for 1998
- HortTechnology, A Comparison of Rapid Potentiometric and Colorimetric Methods for Measuring Tissue Nitrate Concentrations in Leafy Green Vegetables
- USGS, Optical Techniques for the Determination of Nitrate in Environmental Waters: Guidelines for Instrument Selection, Operation, Deployment, Maintenance, Quality Assurance, and Data Reporting