Oxidation Reduction Potential, a Versatile but Misunderstood Wastewater Treatment Monitoring Parameter
Oxidation reduction (redox) reactions are central in water quality management. Biologically mediated redox reactions reduce the oxygen demand and toxicity of polluted water and facilitate the removal of nutrients that fuel the growth of aquatic plants in the environment. In addition, chemically-mediated redox reactions eliminate pathogens from treated liquid effluent and destroy odor-causing compounds in gases released from sewers and treatment processes. A sample of redox reactions that occur in water and wastewater treatment is shown in Table 1.
| Process |
Oxidant |
Reductant |
By-Products
|
|
BOD Removal
|
Oxygen |
BOD (Organic Carbon)
|
CO2(g), water |
| Nitrification |
Oxygen |
Ammonia |
Nitrate (aq) |
| Denitrification |
Nitrate |
BOD (Organic Carbon) |
CO2(g),N2 (g) |
| Dechlorination |
Chlorine |
Sulfite (SO32-) |
Chloride(aq), sulfate(aq) |
| Anaerobic Digestion |
Organic Carbon |
Organic Carbon
|
CO2(g), methane(g) |
| Odor Control |
Chlorine or Nitrate |
Sulfide |
Chloride(aq) or N2(g), sulfate |
| Deammonification |
Nitrite |
Ammonia |
N2(g), nitrate(aq) |
| Taste and Odor Control |
Potassium Permanganate |
Iron and manganese (aq) |
Iron and manganese(s) |
(g) – gas; (s) – solid; (aq) aqueous
The status of redox reactions is vital information for the operation of treatment processes employed to achieve water quality objectives. It is possible to monitor many of the critical redox reactions in water treatment by directly monitoring the increase or decrease of parameters involved in the reactions. However, it may not be practical to monitor all the relevant parameters for every reaction. Alternatively, oxidation reduction potential (ORP) can be used to directly monitor the redox status of the sample environment with a sensor that is easy to operate and relatively inexpensive. The drawback is that the measurement is non-specific, such that it can be complicated to interpret.
Here, we explain the principles of ORP and provide several examples of how it is used to increase the operational efficiency of a water resource recovery facility (WRRF). (Learn more, Biological Nutrient Removal Applications for Monitoring ORP).
Oxidation Reduction Reactions Primer
In order to understand redox, it is first necessary to be familiar with the basic building block of nature, the atom.
A particular atom may have different numbers of electrons. An atom with 3 fewer electrons than protons has a net “charge” of +3 and is said to have an oxidation number of +3. Electrons can also be transferred between atoms resulting in a change in their oxidation number. This is what happens in a redox reaction.
All redox reactions involve an oxidation reaction and a reduction reaction which occur simultaneously. In each reaction, an oxidant gains electrons, and a reductant loses electrons. Think of it as a game of Red Rover, with each child representing an electron. The team sending a child (electron) is the reductant and is oxidized. The team receiving the child is the oxidant (assuming he or she fails to break the chain) and is reduced. A helpful mnemonic is LEO goes GER which means the material that Loses Electrons is Oxidized, and the material that Gains Electrons is Reduced. The classic example is rust, in which the reductant, iron (Fe0), loses electrons and is oxidized to ferric iron (Fe3+) by the oxidant oxygen ( ), which gains electrons and is reduced (O-2), forming iron oxide (Fe2O3).
), which gains electrons and is reduced (O-2), forming iron oxide (Fe2O3).
Of course, not all oxidants and reductants are equal in their ability to receive and donate electrons, respectively. The strength of an oxidant (or reductant) is quantified by its reduction potential. Reduction potential is a measure of the tendency for a substance to be reduced and is influenced by its electron configuration and other properties. The more positive the value, the stronger the oxidant (greater tendency to gain an electron); the more negative the value, the stronger the reductant (greater tendency to lose an electron). Standard Reduction Potential (SRP or E°) is a standard laboratory measurement of the relative oxidizing or reducing the potential of oxidants and reductants. Ozone, permanganate, and hydrogen peroxide have high SRP values indicating their value in chemically destroying pollutants, including pathogens. Oxygen has a relatively high SRT relative to nitrate, demonstrating why bacteria preferentially consume oxygen because they get more bang for the buck.
| Chemical |
SRP (V) |
| Ozone |
+2.08 |
| Permanganate |
+1.70
|
| Hydrogen Peroxide |
+1.76
|
| Chloramine |
+1.45 |
| Chlorine |
+1.42
|
| Oxygen |
+1.23 |
| Nitrate |
+0.94 |
For many reasons, direct measurement of SRP in environmental and process samples is not practical. In those cases, the non-standard parameter ORP provides a simple method for measurement of the net balance of redox reactions in a sample. In general, sample environments with a net balance of oxidized substances, e.g. dissolved oxygen and nitrate, will have a positive ORP, and sample environments with a net balance of reduced substances, e.g. organic carbon and ammonia, will have a negative ORP.
ORP Sensor Design
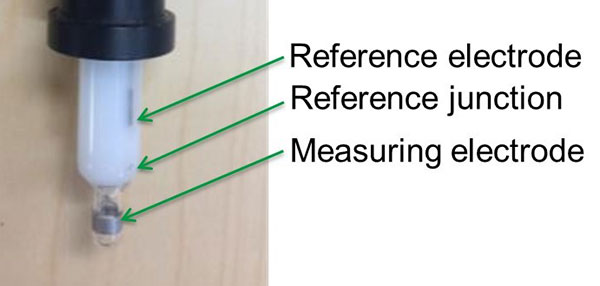
The measurement principle for ORP is potentiometry, a common analytical technique that has been utilized for decades for monitoring pH and many other parameters in water and wastewater samples. ORP is measured as the difference in electrical potential of a measuring electrode in contact with the sample, typically a piece of platinum metal on the outside of the sensor and an internal reference electrode made from Ag/AgCl wire immersed in an electrolyte solution.
The most common design is a combination electrode in which the measuring and reference electrodes are built into one sensor body, such as the IQ SensorNet SensoLyt pH/ORP sensor. A well-designed combination electrode will last for months before needing replacement, with only simple maintenance required. Furthermore, in our experience, calibration is optional in many cases because it is the relative value that matters.
Learn more in our webinar on How pH and ORP Sensors Work or in our webinar Monitoring Oxidation Reduction Potential in Biological Nutrient Removal.
Interpretation of ORP
ORP is widely applicable for monitoring wastewater treatment. Applications include biological processes such as activated sludge and chemical processes such as disinfection.
Biological Treatment
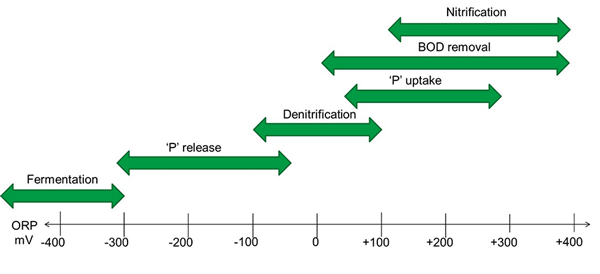
In biological treatment, ORP indicates the presence or absence of DO and nitrate. In the vocabulary of wastewater treatment, the conditions of a biological reactor can be divided into three types:
Oxic is the condition in which DO is non-limiting, which means that organisms that want or need it, get it. In general, ORP is +50 mV and above in an oxic condition. The biological reactions that may occur under oxic conditions:
- Oxidation and removal of organic carbon (as BOD).
- Oxidation of ammonia to nitrate (NO3) by nitrification. BOD removal will precede nitrification. ORP will reach a maximum value after ammonia is depleted, signaling the completion of nitrification.
- Uptake of phosphate by phosphorus accumulating organisms (PAOs). P uptake involves redox reactions, but Phosphorus does not change its oxidation state. PAOs require energy to accomplish P uptake, which is acquired by oxidizing internally stored carbon sequestered during an anaerobic stage with oxygen as an electron acceptor.
Anoxic is the condition in which DO is deficient, but combined oxygen in nitrate (NO3) and nitrite (NO2) are present in amounts to support respiration. ORP is generally +50 mV or less as DO is depleted, and facultative bacteria respire using nitrate instead. The biological reactions that may occur under anoxic conditions:
- BOD removal. If DO is very low, facultative bacteria consume BOD using nitrate, oxidizing BOD to CO2 and nitrate to nitrogen gas.
- Nitrogen removal by heterotrophic denitrification (see the explanation for BOD removal)
- Phosphate uptake. P uptake is not a redox reaction, as stated above. Recent research has revealed that P uptake occurs under anoxic and oxic conditions.
Anaerobic is the condition in which neither DO nor nitrate is present in measurable amounts such that respiration does not occur. In general, the ORP is -50 mV and below in an anaerobic condition. The biological reactions that may occur under anaerobic conditions:
- Phosphate release. Phosphorus does not change oxidation state, but anaerobic conditions are required to select for PAOs which sequester BOD, releasing P in the process.
- Fermentation. Fermentation involves the breakdown of larger organic molecules into smaller organic molecules which are required for PAOs and denitrifying microorganisms. Recent findings have revealed that an ORP of -300 mV or lower is required to achieve the deep anaerobic conditions needed for fermentation.
- Anaerobic digestion.
- Nitrogen removal by autotrophic oxidation of ammonia (Anammox).
Chemical Treatment
Interpretation of ORP in chemical treatment can be very simple when used for monitoring specific reactions to completion. The critical parameter to define is the equivalence point (EP) which defines the concentration of an added chemical at which a reaction has gone to completion. The EP is identified by gradually increasing the chemical dosage until ORP varies dramatically. As chemical dosage is increased beyond the EP, ORP becomes stable again at a higher or lower value than before, depending on the reaction. The figure shows a wide variation in ORP in a chloramine disinfection system on the brink of breakpoint chlorination.
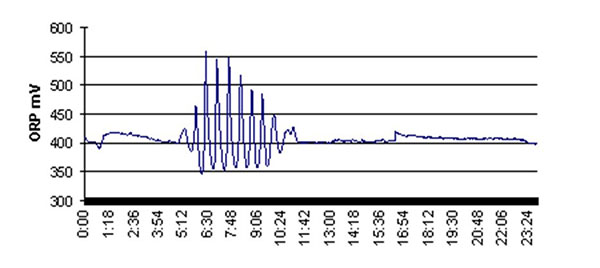
Depiction of a wide variation in ORP in a chloramine disinfection system on the brink of breakpoint chlorination.
Applications for ORP Monitoring in Wastewater
ORP for Activated Sludge Aeration Control
Among the chief goals for modern WRRFs are nutrient removal and energy conservation. The traditional DO target concentration of 2.0 mg/L, grounded in scientific and empirical evidence, is based on making oxygen non-limiting to provide the most efficient removal of BOD and ammonia from wastewater. From the perspective of a bacterium, obtaining reduced power from oxygen provides the highest return on investment. However, this approach consumes a lot of energy.
Furthermore, it also limits the diversity of the mixed liquor ecosystem. On the other hand, when oxygen is limiting, energy efficiency and bacterial diversity are increased. Therefore, some WRRFs seek innovative ways to achieve nutrient removal while minimizing energy consumption.
ORP is very useful in batch treatment systems like sequencing batch reactors (SBR) and aerobic digesters. ORP variation signals key endpoints in the nitrification and denitrification processes. A change in slope occurs at the point of ammonia and nitrate depletion, as shown in the hypothetical example. This information can be used to control aeration on-off cycles. (Learn more, ORP Management in Wastewater as an Indicator of Process Efficiency).
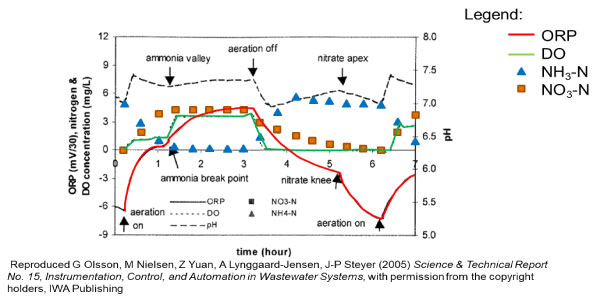
The Fairborn Water Reclamation Center automated aerobic digester operation based on ORP. One of the side benefits was reduced operations and maintenance labor for the final clarifiers. The nutrients in the dewatering filtrate would pass through main-stream treatment to the final clarifiers and stimulate algae growth on weirs and structural steel in the relatively still, clear water. The algae is unsightly and would have to be manually cleaned at regular intervals or else risk effluent violations for Total Suspended Solids (TSS). The cyclic aeration control depicted in the accompanying figure is described below:
- Start aeration at the beginning of the day and aerate continuously until ammonia breakpoint, as indicated by ORP, is reached
- Aerate intermittently during the day to keep solids in suspension and provide a consistent feed for dewatering
- Stop aeration at the end of the day
- Start aeration overnight when ORP reaches a pre-set minimum.
Fairborn has since upgraded the aerobic digestion system, but ORP remains the basis for aeration control.
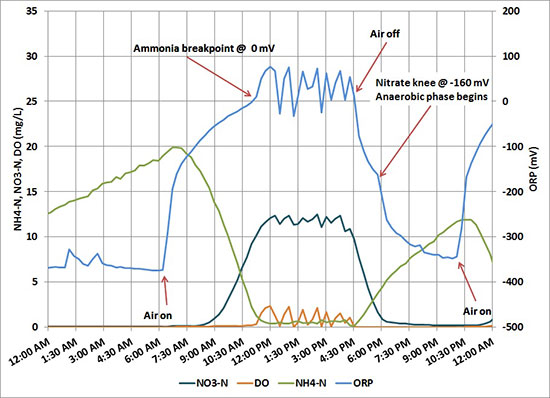
Cyclic aeration control at the Fairborn Water Reclamation Center.
The Fairborn example illustrates another key feature of ORP. A strategy based on ORP also allows for the implementation of enhanced biological phosphorus removal (EBPR). The nitrate knee, besides signaling the depletion of nitrate, also initiates anaerobic conditions in which phosphate-accumulating organisms (PAOs) release internal phosphate. Therefore, phosphate release is enabled by delaying the start of the aeration ‘on’ period following the nitrate knee (more on EBPR to follow).
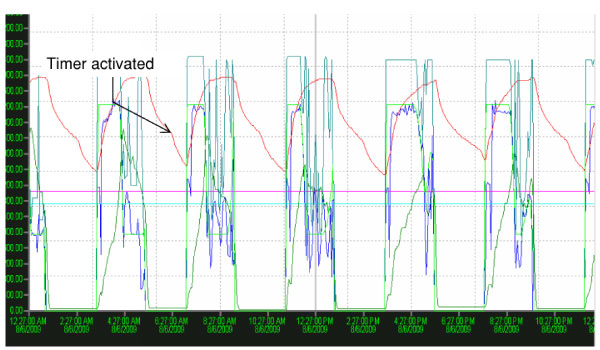
ORP is also the heart of the aeration control strategy for Schreiber CSR (Continuously Sequencing Reactor) biological phosphorus removal system at the Greene County Sugarcreek Water Reclamation Facility. A 40-minute ‘watchdog’ timer is activated as ORP passes through the nitrate knee, facilitating phosphate release. Aeration resumes as the timer expires and phosphate uptake occurs. The strategy has demonstrated that an effluent TP of less than 0.8 mg/L can be consistently achieved (Smith and Goble, 2010).
ORP is also useful for continuous flow systems. For example, the energy efficiency of activated sludge systems can be increased by operating at DO concentrations less than 1.0 mg/L. A YSI customer in Illinois experienced an 80% reduction in aeration energy consumption due to an aeration improvements project that included a new automated aeration control system based on online measurements of DO and ORP (A. Poole, personal communication, 2013).
Nutrient removal is also enabled at low DO through simultaneous nitrification and denitrification (SND). An ORP sensor is a good supplement, or even replacement, for DO in this application because it provides more information. Whereas a DO sensor measures oxygen concentration, an ORP sensor measures the net effect of all relevant species, including nitrate. Furthermore, ORP also provides higher resolution and may vary from -100 mV to +100 mV in a region where DO is limited from 0.1 to 1.0 mg/L
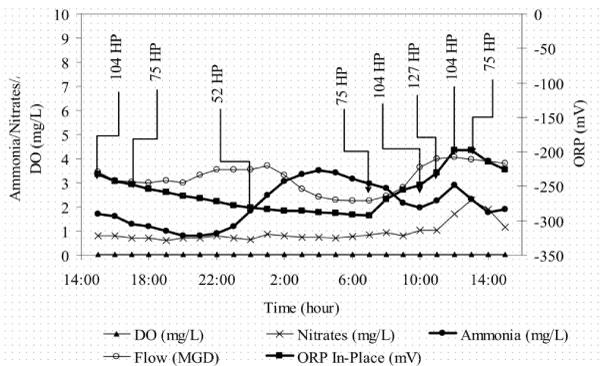
SND is a well-documented feature of oxidation ditch technology. Therefore, it is not surprising that ORP is often used to automate aeration control in oxidation ditches. A good example is presented in the chart below (Myers, et al, 2006). Nitrate concentrations less than 5 mg N/L are achieved with the automation of each of two 2-speed mechanical aerators. ORP tracks the variation in ammonia while the measured DO concentration is constant at the detection limit. The location of the ORP sensor between the aerators is a critical aspect of the strategy. The ORP signal would be dominated by DO at locations just downstream from the aerator, masking variations in other important parameters like ammonia and nitrate.
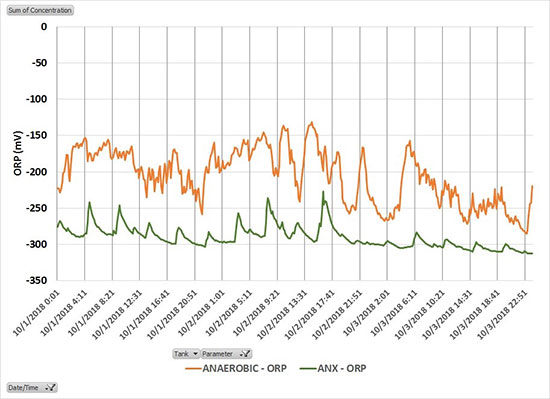
Recent discoveries into the diversity of PAOs are the driving force for designing more reliable EBPR processes. Weak wastewater and wet weather are a problem for conventional EBPR, which relies on raw wastewater as a carbon source. Alternatively, an internal source of readily degradable carbon can be created by passing a side-stream of mixed liquor or RAS through an offline or inline fermentation zone, a technology known as side-stream EBPR (S2EBPR). ORP provides a measurement of the reactor conditions needed for S2EBPR to succeed. Deep anaerobic conditions, as indicated by an ORP of -300 mV or less have been found to favor the growth of PAOs with desirable metabolic capabilities.
For example, Tetrasphaera spp. that ferment higher carbon forms (not just VFA), produce VFA, which can be used by other PAOs, and take up phosphorus under anoxic conditions (Barnard, et al 2017). The Bradford, Ohio WWTP modified its conventional EBPR process to create an inline fermentation zone upstream from an oxidation ditch. The original anaerobic zone serves as a preanoxic zone to denitrify RAS prior to entering the “anoxic” zone. Anoxic zone mixers are operated intermittently a total of 3 hours each day and the nitrate internal recycle gate into the anoxic zone is closed. An IQ SensorNet ORP sensor installed 5 ft. below the water level in each of the former anaerobic (upstream), and anoxic (downstream) tanks indicate fermentative conditions. Monthly average effluent Total Phosphorus (TP) averaged 0.54 mg P/L over the last 16 months with values as low as 0.18 mg P/L without the use of chemicals (vanDommelen and Roberts, 2020).
ORP for Disinfection Control
Chemical disinfection processes are used in wastewater treatment to destroy pathogens. In general, a high ORP value is desired to ensure the desired pathogen kill is achieved. The target value depends on the chemical oxidant used and can be determined through trial and error. If chlorine is the disinfectant, the excess must be removed before it can be discharged. This is typically achieved with bisulfite. The target ORP is determined by observing the dosage at which an abrupt decrease in ORP is observed, signaling the point that residual chlorine is consumed and the water is in compliance.
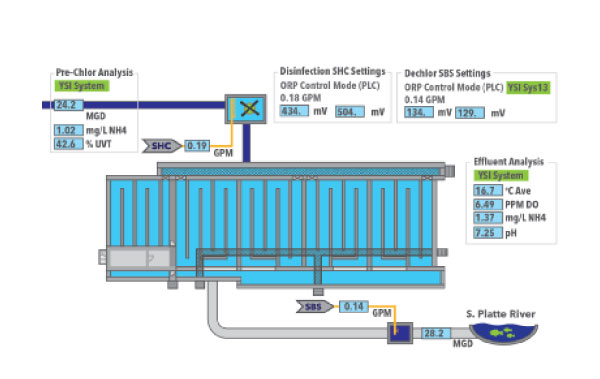
The South Platte Water Renewal Partners WRRF in Englewood, CO, utilizes chloramine disinfection by dosing chlorine to secondary effluent containing ammonia. The disinfecting power of chloramines depends on the types of chloramines formed, complicating the use of total residual chlorine for process control. ORP, on the other hand is a measure of disinfecting power. Chlorine dosage is controlled to achieve a target ORP at a sensor downstream from the chlorine injection point. The target ORP is optimized to achieve the desired pathogen kill while preventing the onset of breakpoint chlorination, which would result in higher chlorine consumption. A second ORP monitoring system downstream from the sodium bisulfite injection point controls dechlorination. Sodium bisulfite dosage is controlled to achieve a target ORP which assures that all unreacted chlorine is consumed. A unique feature of the monitoring system is that a redundant ORP sensor is installed at each of the upstream and downstream monitoring locations.
Summary
The examples provided demonstrate the versatility of the ORP parameter for monitoring and controlling wastewater treatment. ORP is useful for monitoring biological and chemical processes. In biological processes, ORP provides more information than DO, especially when the DO concentration is low. In chemical processes, ORP can provide a clear indication of when reactions have been completed.

References
Barnard, J.L., Dunlap, P., and Steichen, M., “Rethinking the Mechanisms of Biological Phosphorus Removal”, Water Environment Research 89(11), 2017.
Myers, M., Myers, L, Okey, R., “The use of oxidation-reduction potential as a means of controlling effluent ammonia concentration in an extended aeration activated sludge system”, WEFTEC 2006.
Smith, R.C., and Goble, L., “To Everything There Is a Season; Lessons from Four Seasons of Phosphorus Removal at Greene County Sugarcreek WRRF”, WEFTEC 2010.
vanDommelen, J. and Roberts, J., “Kicking the Chemical Habit: Operational Modification of a “BNR” Oxidation Ditch for Biological Phosphorus Removal”, Ohio WEA Technical Conference & Exhibition, 2020.