Tips, Cautions, and Limitations for ORP Measurements
ORP (oxidation reduction potential or redox) is typically measured to determine the oxidizing or reducing potential of a water sample. It indicates possible contamination, especially by industrial wastewater. ORP can be a valuable measurement if the user knows a particular component within the sample that is primarily responsible for the observed reading. For example, excess chlorine in wastewater effluent will result in a large positive ORP value and the presence of hydrogen sulfide will result in a large negative ORP value.
ORP is determined by measuring the potential of a chemically inert (platinum) electrode which is immersed in the solution. The sensing electrode potential is read relative to the reference electrode of the pH probe and the value is presented in millivolts (mV).
The determination of ORP is particularly worthwhile in water which contains a relatively high concentration of a redox-active species, e.g., the salts of many metals (Fe2+, Fe3+) and strong oxidizing (chlorine) and reducing (sulfite ion) agents.
Thus, ORP can sometimes be utilized to track the metallic pollution in-ground or surface water or to determine the chlorine content of wastewater effluent. However, ORP is a nonspecific measurement, i.e., the measured potential is reflective of a combination of the effects of all the dissolved species in the medium. Because of this factor, the measurement of ORP in relatively clean environmental water (ground, surface, estuarine, and marine) has only limited utility unless a predominant redox-active species is known to be present. Users should thus be careful not to “over-interpret” ORP data unless specific information about the site is known.
ORP vs. Eh: Calibration of ORP Sensors
Many users of YSI instruments, such as the EXO sonde, ProDSS, or ProQuatro handhelds that make field or laboratory redox measurements have questions about the difference between ORP and Eh.
In essence, the two parameters are the same in that both quantify the potential of the medium to transfer electrons -- the difference is the reference electrode (and thus the voltage offset) against which the potential of the platinum sensor is reported. Eh is defined as a voltage reading vs. the Standard Hydrogen Electrode (SHE), while ORP is a much less specific term in which the measurement can be made relative to any practical or theoretical reference electrode, such as Ag/AgCl, calomel, or SHE. Generally, it is not easy to use the SHE in laboratory or field measurements and thus redox readings are made using either the Ag/AgCl or calomel reference electrodes, with Ag/AgCl being more popular in multiparameter water quality instrumentation.
Thus, Eh is usually not determined directly. However, the voltages obtained as ORP readings vs. non-SHE electrodes can easily be converted into Eh values by two mechanisms.
Adding (or subtracting) an offset voltage to the ORP readings obtained vs. a practical reference electrode to account for the fixed difference between the SHE and the other reference system. The offset voltage can easily be obtained for several practical reference electrodes in Table 2580: II of the section of Standard Methods on ORP. For example, the potential of Zobell solution vs. the Ag/AgCl reference electrode using 4 M KCl is +228 mV while the same solution read vs. the SHE is +428 mV. Therefore to convert ORP readings taken under these conditions to Eh, simply add 200 mV to the ORP voltage. For example, ORP readings of +150 and -172 mV translate to Eh values of +350 and +28 mV, respectively.
Effect of Temperature on ORP
The temperature of the water for which ORP is being determined will affect the voltage output of the sensor. This factor definitely needs to be taken into account for calibration and should be considered when reporting field ORP values.
For calibration, the following table can be used when using Zobell solution, the YSI recommended standard. Thus, if the Zobell calibration standard is at 15°C instead of 25°C, enter 241 mV at the calibration prompt instead of 228 mV (the 25°C value which is commonly quoted).
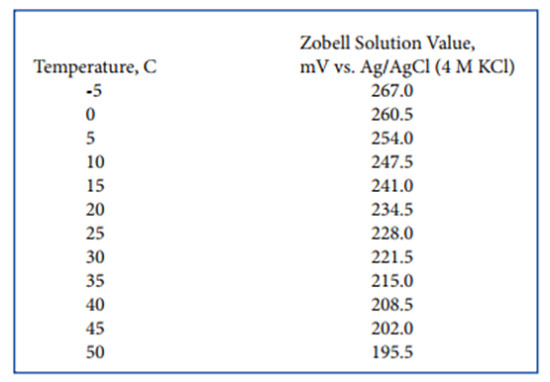
The user may be able to locate similar temperature-dependence data in the literature for other ORP standards such as Light’s Solution and quinhydrone standards in pH buffers
Problems with ORP Sensors
Although based on relatively simple theory, ORP is, unfortunately, also a measurement that can show more problems than other water quality sensors with regard to consistency between different instruments and overall accuracy. In addition, these issues are further complicated in that their extent is likely to depend on both the condition of the sensor and the makeup of the water being tested.
The most common problem reported with regard to ORP determination in environmental water is that readings from various instruments (sometimes with exactly the same sensor type and electronics) differ by a significant margin (50-100 mV) even though the sensors are in the same container of water. To make the problem more perplexing, all of the sensors show identical readings in an ORP standard such as Zobell solution. The exact explanation for this paradox is sometimes elusive, but there are at least three possible reasons for its occurrence.
- ORP sensors can show a slow response in environmental water if the platinum button of the probe has been contaminated with extraneous material. Common contaminants include hard water deposits, oil/grease, or other organic matter. If the platinum electrodes in the above example are variably contaminated, then some of them (the more contaminated) will be likely to approach potentiometric equilibrium slower. Under this scenario, if left long enough, all the sensors would read the same. However, it might take days for the contaminated sensors to reach their final value, and, therefore, they appear in the time frame of a sampling experiment (< 1 hour), to be different. Naturally, if the electrode contaminant is redox-active, either in itself or because it contains redox-active impurities, the reading from that sensor will exhibit erroneous readings that may never change unless the contaminant is removed.
- Users often see discrepancies when comparing multiple ORP sensors. One factor contributing to this is clean environmental water. There may be very few redox-active species present, and those that are present may be in very low concentration. In many cases, the concentration can be so low that the redox influence of the species is effectively below the detection limit of the method. Under these conditions, the readings will have questionable meaning and could show this type of variation described above. Note that the ORP reading variance associated by this scenario is likely to be exacerbated if any of the electrodes is also contaminated as described above.
- The makeup of the surface composition of the electrode may not be ideal for the measurement in the medium under investigation.
The fact that similar or identical ORP sensors read differently in environmental water yet the same in Zobell solution is due to the fact that the concentration of redox-active species (ferricyanide/ferrocyanide for Zobell) is much greater in the standards.
This higher concentration usually “swamps out” the inconsistencies related to detection limit problems (caused by low amounts of redox-active species) and response time issues (caused by electrode contamination), thus all sensors respond rapidly and read within the YSI specification of +/- 20 mV when in standards. If you observe inconsistency between different sensors or experience ORP readings which seem unusual for the water being tested with your YSI instrument, YSI recommends the following steps to identify and/or correct the problem:

First , make certain that the pH sensor is functioning properly. If you are utilizing a combination pH/ORP sensor the reference electrode is common to both the pH and ORP sensors and, therefore, if both pH and ORP sensors are malfunctioning it is likely a failed reference is the source of the problem. Reference electrode problems usually appear as either total failure or as a slow response in both pH and ORP readings. If a reference electrode problem is suspected, test the ORP sensor in a standard and make certain that it is within 20-30 mV of the predicted value. If reference electrode performance is indicated, clean the sensor according to the instructions shown below and then retest.
Second, if the sensor performs well in the ORP standard, remove the probe from the instrument and carry out the sequential cleaning process documented in the next section.
ORP Electrode Cleaning
The following procedure will result in the removal of many common contaminants from the platinum ORP electrode. Fouling of the electrode can be deployment-specific, and some contaminants from polluted water may not be dissolved by this method. The use of other solvents and reagents may be possible, but they must be selected carefully so as not to damage the reference electrode or pH glass of the combination sensors nor to leach or dissolve the body of the probe itself. Consult YSI Technical Support before using cleaning methods other than those documented below. YSI recommends that the user perform the cleaning/reconditioning operation in the order indicated. Performance can be rechecked at the conclusion of each major section (A, B, and C), and the cleaning discontinued if, at that point, the performance problem has been corrected.
Procedure A
- Soak the probe for 10-15 minutes in clean water containing a few drops of commercial dishwashing liquid.
- Wipe the platinum button or ring by rubbing with a cotton swab soaked in the cleaning solution. CAUTION: Be certain not to damage the glass bulb of the combination sensor during this process. Any pressure on the glass bulb can cause breakage.
- Rinse the probe in clean water, wipe with a cotton swab saturated with clean water, and then re-rinse with clean water.
Procedure B
- Soak the probe for approximately 1-2 hours in up to a 1:1 dilution of commercially available chlorine bleach.
- Rinse the probe with clean water and then soak for at least 1 hour in clean water to remove residual bleach from the reference junction. CAUTION: If removal of the chlorine bleach is incomplete, this cleaning reagent can seep into either your calibration standards or measurement media and cause erroneous ORP readings until it is dissipated. Always err on the side of caution in the chlorine bleach removal. Soaking the probe in clean water for periods of time longer than 1 hour can do no harm, however, lesser soaking times can cause problems.
Procedure C (Necessary only if hard deposits are present such as barnacles.)
- Soak the probe for 20-30 minutes in one molar (1 M) hydrochloric acid (HCl). This reagent can be purchased from most laboratory supply dealers. Be sure to follow the safety instructions supplied with the reagent.
- Wipe the platinum button by rubbing with a cotton swab soaked in the acid. CAUTION: Be certain not to damage the glass bulb of the combination sensor during this process.
- Rinse the probe in clean water, wipe with a cotton swab saturated with clean water, then rerinse with clean water.
- Dry the probe’s port and probe connector with compressed air and apply a very thin coat of O-ring lubricant to all O-rings before re-installation of the probe. After the probe is reinstalled, place the sensors in Zobell solution and make certain that observed ORP readings stabilize within a few minutes and remain stable for 15-20 minutes.
Summary
The determination of ORP in environmental water can provide valuable insight into the sample as long as there is a significant concentration of a redox-active species present. However, in the absence of these species, ORP can be a significantly less exact measurement than many other sensors. The inexactness is usually due to contamination of the electrode surface (either physically or chemically), but can also be due to the lack or low concentration of redox-active agents in the environmental water.
The quoted accuracy specification for YSI ORP sensors (+/- 20 mV) refers to redox- standards, such as Zobell solution, and not to environmental water of variable, and usually unknown, content. In many cases, the +/- 20 mV specification will be met in natural water, but it cannot be guaranteed.
Periodic maintenance of your YSI ORP sensors will increase your field consistency and accuracy, but may not overcome all problems.
The value of ORP in determining the content of environmental water is greatly enhanced if the user has some knowledge or history of the site. ORP data can typically become more useful if used as an indicator over time and/or with other common parameters to help develop a complete picture of the water quality being tested.
Historic data can be used to help determine the validity of the data or a proper functioning probe. But inconsistent data does not always indicate a bad or dirty probe. There could be environmental factors contributing to the unexpected data.
Questions? Please contact our Technical Support group for any ORP related questions.
Additional Blog Posts of Interest
Laboratory Instruments and Electrodes for pH, ORP and ISEs with YSI TruLab
How ORP Sampling Helped Determine Arsenic in Drinking Water Wells
ORP Management in Wastewater as an Indicator of Process Efficiency
The Evolution of Water Quality Monitoring [Free EBook]