Online Instrumentation for Chlorine Disinfection Control in Wastewater
Chlorination has long been an effective strategy for disinfection in municipal wastewater. Although alternative forms (UV and ozone) are becoming more efficient and widely used, chlorine disinfection remains the primary approach to dealing with pathogenic organisms. Potentially harmful microorganisms in wastewater effluent must be treated to avoid impacting humans and animals. Chlorine is useful as a disinfectant because it is a strong oxidant that can break down the cell walls of pathogens and either deactivate or kill them.
Since chlorine is such a strong oxidant, discharged water with high concentrations can harm plants and animals within the ecosystem. Also, high chlorine concentrations in the presence of organic compounds can form DBPs and can be detrimental to human health. For these reasons, chlorine control strategies using online instrumentation are vital to ensure just the right amount of chlorine dosing to achieve the desired amount of disinfection.
Additionally, chlorine control strategies can save on operating costs. Ordering, delivering, and storing chlorine can be expensive, so optimum efficiency is critical when dosing. Online instruments provide the continuous measurements required to control chlorine dosing based on a set point. YSI manufactures two online instruments for chlorine disinfection control, the 3017M DPD Chlorine Analyzer and the SensoLyt ORP sensor. This blog will discuss chlorine disinfection in wastewater and how these instruments can control chlorine dosing.

The YSI 3017M DPD Chlorine Analyzer and IQ SensorNet SensoLyt ORP Sensor.
The Disinfection Process
The disinfection stage of wastewater treatment typically occurs after secondary treatment and just before the final effluent. Chlorine is introduced to the wastewater stream at the beginning of the contact basins within a mixing chamber to ensure a uniform reaction between the process water and chlorine. Several forms of chlorine can be utilized, such as elemental chlorine (Cl2), sodium or calcium hypochlorite (ClO-), or chlorine dioxide (ClO2). Chlorine reacts with water and its other constituents to create several chemical compounds, such as free chlorine (Hypochlorous acid) and combined chlorine (monochloramine, dichloramine, trichloramine), or it reacts with the organic/inorganic content within the water.
The chlorine contact basins typically have a plug flow design, in which the chlorinated water flows through the basins and allows the chlorine time to react. Contact time and chlorine dosage are the two primary control factors to attain the desired amount of disinfection. Still, other factors like pH, temperature, BOD, TSS, ammonia, and nitrite can alter the effectiveness of chlorine. Dechlorination is required in many wastewater systems before discharge because residual chlorine can be toxic to organisms in natural waterways, even at small doses. For dechlorination to occur, an additional chemical, such as sodium bisulfite or sulfur dioxide, is dosed to the system reducing the total chlorine residual to a safe level.
Chlorine Dosing
Before discussing dosing, the behavior of chlorine in wastewater must be understood. When added to the contact tanks, chlorine first reacts with the organic matter and other contaminants referred to as the chlorine demand. As chlorine is added at higher rates, chlorine also reacts with any ammonia in the water, creating combined chlorine, which adds to the total residual chlorine (Table 1). As dosing increases and all available ammonia has reacted, free chlorine residual begins to form (HOCL). This point is known as breakpoint chlorination and the stage in which there is enough chlorine dosed to disinfect with free chlorine (Figure 1).
|
Forms of Chlorine in Breakpoint Chlorination
|
Description
|
|
Free Chlorine
|
The available free chlorine for disinfection in the forms of hypochlorite ions (OCl-) and hypochlorous acid (HOCl), depending on the pH.
|
|
Combined Chlorine
|
Chlorine that has combined with ammonia to form chloramines, including monochloramine, dichloramine, and trichloramine.
|
|
Total Chlorine Residual
|
The total amount of chlorine present, including both free chlorine and combined chlorine.

|
Table 1: Description of forms of chlorine in the breakpoint chlorination curve, showing the relationship between Total, Free, and Combined Chlorine.
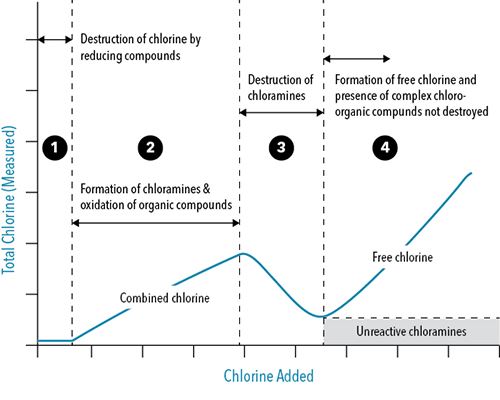
Figure 1: Breakpoint Chlorination Curve, which compares the behavior of free and combined chlorine as chlorine dosing is increased in a system. The description of each stage is below:
- When adding in low doses of chlorine, the chlorine will first react with reducing compounds, which are typically inorganic matter in the water.
- Increasing dosing more, chlorine begins to react with organic compounds and ammonia. The chlorine and ammonia form monochloramine, which increases the total chlorine.
- After all ammonia has reacted with chlorine, monochloramines will be converted to di- or trichloramines, which reduces the total chlorine measured.
- There is enough chlorine present to satisfy the chlorine demand (inorganic matter, organic matter, ammonia) and free chlorine is able to form.
Free chlorine is the most effective disinfectant, so many facilities will dose enough chlorine to surpass breakpoint chlorination. A free chlorine residual of 0.2 mg/L is typically enough to ensure sufficient disinfection and meet fecal coliform limits. Combined chlorine can also be used as the disinfectant depending on the system's design, although it presents a weaker disinfectant capability. Depending on the amount of ammonia in the water and the requirements of your facility, it may make sense to disinfect with combined chlorine rather than free chlorine. For example, in systems with consistently higher ammonia levels (1-5 mg NH4-N/L), the amount of chlorine needed to reach past breakpoint chlorination would be challenging to maintain, thus aiming for monochloramine formation is more reasonable.
The amount of chlorine dosed must be enough to satisfy the chlorine demand and ammonia to reach breakpoint chlorination for free chlorine residual. If the required chlorine dose is known, it can be converted to a feed rate signal, which tells the chlorinator the rate to add chlorine to the wastewater stream. Chlorine dosage can be calculated by adding the calculated chlorine demand and chlorine residual and then be converted to a feed rate by multiplying with the flow (MGD) and 8.34 (density of water) (Figure 2). The chlorine feed rate can be automatically controlled using a YSI 3017M DPD Chlorine Analyzer or a YSI SensoLyt ORP sensor.

Figure 2: Both chlorine demand and chlorine residual can be calculated in the first equation with instrumentation and an associated chlorine dose at the time. The resulting chlorine doses can then be calculated and input into the second equation for calculation of the chlorine feed rate.
Process Control with the 3017M DPD Analyzer
Process control is the automated control of an action based on the value of a process variable. With chlorination, the feed rate can be automatically adjusted based upon either a chlorine residual or ORP measurement. Feedback control systems are most common when using free or total chlorine analyzers, with the analyzers downstream of the dosing point and after the contact basins (Figure 3). (Learn more, Wastewater Process Control - Which Strategy is Right for You?).
Based on the current measurement, the downstream analyzer controls the chlorinator based on a set point, either increasing or decreasing chlorine dosing. Both a free and total chlorine analyzer are recommended to most effectively control dosing with the 3017M. Together, these two measurements can provide the information needed to control chlorine dosing, determine combined chlorine residual, and provide the operators with their location on the breakpoint chlorination curve. If measuring both free and total chlorine is not an option, it may be possible to control chlorine dosing with a single analyzer, depending on the conditions of your application. For example, in applications with near-zero ammonium, combined chlorine will only exist in small concentrations. Therefore, total and free chlorine concentrations would be the same, allowing you to control with either value.
Over the 100-year history of chlorine use in water disinfection, several analytical methods have been developed to determine chlorine concentration. The 3017M utilizes the DPD colorimetric method (N, N-diethyl-p-phenylenediamine), in which the sample is combined with a buffer reagent and the DPD indicator solution for measurement of free chlorine. An additional potassium iodide solution is added to the indicator solution for total chlorine measurement, allowing the chloramines to react with DPD. The resulting reaction creates a pinkish color, in which the absorbance is proportional to the amount of free or total chlorine in the sample.
The 3017M utilizes flow injection analysis, meaning a continuous flow of sample is injected with the DPD indicator solution and buffer. The sample and reagents mix on their way to the photometer, where the absorbance of the sample is measured at 525 nm. Flow injection analysis in the 3017M accomplishes mixing and measurement without moving parts, reducing maintenance and hardware malfunctions compared to other wet chemistry analyzers. In addition, the 3017M has an adjustable measuring interval, meaning it can be programmed to measure in the range of every 2.5 to 60 minutes.
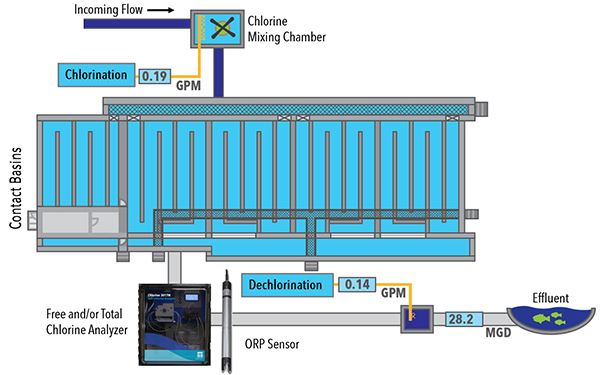
Figure 3: A diagram showing the placement of the 3017M DPD Chlorine Analyzer and SensoLyt ORP sensor within a wastewater disinfection process. The typical placement for the instrument is after the contact basins, which then provide a feedback signal for chlorine dosing. A similar feedback system can be used for dechlorination by utilizing a DPD Chlorine Analyzer and SensoLyt ORP sensor near the final effluent.
Process Control with the SensoLyt ORP sensor
Controlling chlorine dosing with ORP has advantages and disadvantages compared to DPD chlorine analyzers. ORP, or oxidation-reduction potential, measures the oxidizing capability of the solution rather than the amount of chlorine residual. Since the oxidizing capability of the sample is directly related to its ability to disinfect the pathogens in the water, ORP is an effective control parameter for chlorine dosing. Operators can determine an ORP set point in which they are confident they are sufficiently disinfecting the water and then control dosing to this set point. For example, wastewater treatment facilities can maintain ORP values between 500 and 800 mV to ensure they achieve the desired disinfection (Figure 5). However, the disadvantage of ORP is that this is a non-specific, indistinct measurement. Operators must perform analytical work to determine the correct ORP value as the set point, which can be different between facilities. Additionally, since ORP is an indistinct measurement, the sensor cannot provide concrete chlorine concentrations and thus less valuable information than the DPD analyzers. (Learn more, ORP Management in Wastewater as an Indicator of Process Efficiency).
Control strategies with ORP are typically feedback systems. In this case, the ORP sensor would be placed downstream of the chlorine feed location and dose chlorine automatically to maintain the desired set point. ORP utilizes a combination electrode to measure the oxidation-reduction (redox) potential. Depending on the types of oxidizing or reducing molecules in the water, redox reactions (chemical or biological) are more likely or less likely to occur. Chlorine, for example, is a strong oxidizing chemical and increases the ORP value.
When more chlorine is present, there will be an increase in ORP, and thus a higher likelihood that the pathogens in the water will be killed or deactivated. The YSI SensoLyt ORP sensor is a robust and reliable option for online ORP monitoring. Electrodes are user-replaceable without extra tools, meaning no electrolyte or salt bridge replacement. Each electrode is backed with a 6-month warranty and is expected to last over 12 months before replacement. Both the SensoLyt ORP sensor and 3017M DPD Chlorine Analyzer can be easily integrated into IQ SensorNet. YSI’s IQ SensorNet is a network of modules and sensors that can be configured to monitor critical wastewater parameters across a facility with a single system.

YSI IQ SensorNet System Controllers.
Conclusion
The correct online instrument for your facility may depend on several factors. The 3017M DPD Chlorine Analyzer provides tangible, accurate information on chlorine residuals for controlling chlorine dosing and monitoring breakpoint chlorination. On the other hand, the SensoLyt ORP sensor offers a measurement of the oxidizing capability of the water that can control chlorine dosing based on the ORP set point. Compared to wet chemistry analyzers, ORP sensors are smaller and more affordable, but ORP does not provide concrete chlorine residual numbers. The 3017M DPD Chlorine Analyzer and the SensoLyt ORP sensor are great options to automatically control your chlorine dosing to ensure you are sufficiently disinfecting and preventing overfeeding. However, the best online instrument for your disinfection process depends on the needs of your facility and the operators maintaining the system.
Want to learn more?
Visit our resource pages to learn more about the 3017M DPD Chlorine Analyzer, SensoLyt ORP sensor, and IQ SensorNet system of online sensors and controllers for wastewater process monitoring and control.
Or check out our on-demand webinar, below.