Expert Tips on Chlorine Measurement: Understanding Free vs. Total Chlorine
Chlorine is a powerful disinfectant used in municipal water treatment processes for over 100 years. From its first use in 1908 in New Jersey to now, it has become the primary disinfectant used in most drinking water and wastewater plants in the United States.1
Chlorine disinfects water by killing or deactivating many pathogens that lead to disease. Some of the well-known waterborne diseases that chlorine neutralizes are E. coli, Hepatitis, and Giardia. Chlorine-based disinfectants provide a more potent residual effect than other disinfectants; therefore, they play a vital role in a municipality’s distribution network and remain the disinfectant of choice for drinking water plants. In this article, we’ll explore the differences between free chlorine and total chlorine and discuss the applications in which each should be measured.
Raw water versus tap water with chlorine disinfection
How Chlorine is Used as a Disinfectant at Water Treatment Plants
To determine when free versus total chlorine should be measured, we first need to discuss the differences between the two parameters and explain the basics of chlorine disinfection in municipal treatment plants.
Free chlorine refers to the amount of chlorine in the water available to sanitize and kill harmful bacteria, viruses, and other pathogens. This type of chlorine is the most reactive form, meaning it is available to react with and neutralize contaminants in the water. In municipal water plants, free chlorine is often dosed in the form of chlorine gas, which forms either hypochlorous acid (HOCl) or hypochlorite ions (OCl-) when added to water.
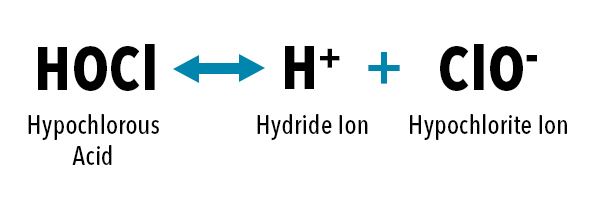
Equilibrium equation for hypochlorous acid in water
As free chlorine is dosed into municipal water, it reacts with inorganic and organic compounds such as ammonia and nitrate. Free chlorine breaks down the cellular structures of these contaminants, producing new compounds called chloramines, including monochloramine, dichloramine, and trichloramine. In general, these compounds are referred to as combined chlorine.
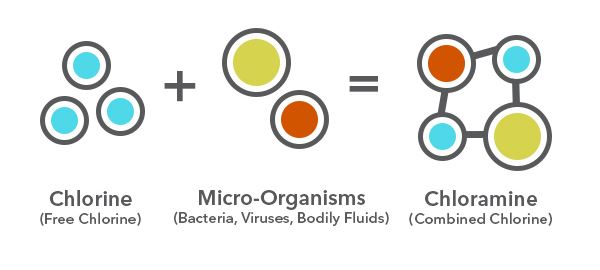
Chloramine compounds form from the reaction of free chlorine and micro-organisms. Adapted from: poolonomics.com
In some cases, chloramine is used to disinfect drinking water, offering a few advantages compared to conventional free chlorine disinfection. Chloramines are a slightly weaker disinfecting compound than free chlorine due to the fact they are partially reacted with ammonia. As a result, chloramine produces less disinfection byproducts when it reacts with contaminants in the water.
Disinfection byproducts (DBPs) are harmful compounds known to cause adverse effects to animals and humans if consumed, including the formation of various cancers. Although there are many types of DBPs, some of the more common and well-known include trihalomethanes (THMs), chloroform, haloacetic acids (HAAs), chlorite, and bromate. Additionally, chloramine compounds are more stable than free chlorine compounds and are thus able to disinfect for longer in drinking water applications. This is particularly helpful when a municipality has a large distribution network where the water has a long travel time to the end user. In some cases, plants use a free chlorine strategy at the plant and a chloramine dosing strategy in the distribution network.
Total chlorine is the sum of free chlorine and combined chlorine and is crucial in helping to give a full picture of how much chlorine is in a water sample. When free chlorine is subtracted from total chlorine, we can find the concentration of combined chlorine.
Now that the basics of chlorine as a disinfectant have been covered, we can apply this knowledge to determine when to measure free chlorine, total chlorine, or both in the various control strategies used in wastewater and drinking water applications.
Chlorine in Wastewater
When it comes to municipal wastewater treatment, total chlorine is typically measured. Wastewater plants primarily discharge water back into the environment, with effluent water being discharged into lakes, rivers, and streams. Wastewater plants have discharge permit requirements on total residual chlorine to ensure the receiving water body will retain a safe chlorine level since any form of chlorine can be highly toxic to aquatic life.

Wastewater treatment plant
When measuring the chlorine concentration in the effluent of a wastewater treatment plant, it is important to measure the total chlorine content because this measurement accounts for both free and combined chlorine. Chlorine chemicals can be expensive to buy and store, so ensuring that the chlorination process is as efficient as possible is a high priority for plant operators. Utilizing instrumentation that measures chlorine and other parameters, including pH, temperature, BOD, TSS, ammonia, and nitrite, can optimize the chlorination process at wastewater treatment plants.

Wastewater effluent
The chlorination process can be controlled in several ways, but the most common control strategy is breakpoint chlorination. This is a process where chlorine is added until the breakpoint is achieved. The breakpoint is when all organic matter in the process has reacted with chlorine to form combined chlorines. After the breakpoint is achieved, additional chlorine may be added to get to the desired residual chlorine amount before the dechlorination process.
Breakpoint chlorination is described in more detail in our Online Instrumentation for Chlorine Disinfection Control in Wastewater blog. Measuring total and free chlorine during the chlorination process allows the operator to derive the combined chlorine residual, giving a full picture of the process. This data can help operators efficiently dose enough chemicals to get a sufficient disinfection rate without the potential of overdosing and wasting chemicals.
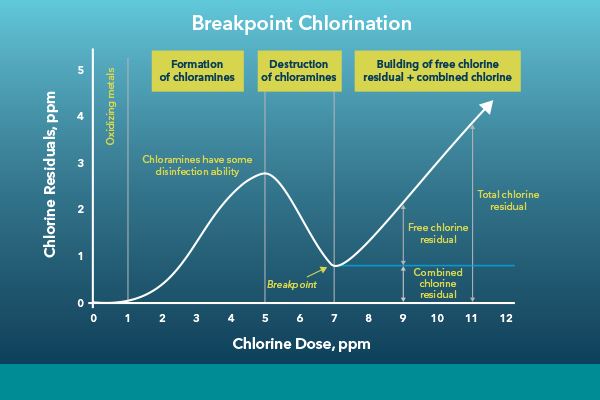
Breakpoint chlorination in wastewater treatment. Adapted from: Orenda Technologies, https://blog.orendatech.com/breakpoint-chlorination-explained
Chlorine in Drinking Water
Maintaining a minimum chlorine residual is vital in drinking water as it ensures that drinking water effluent remains disinfected when it reaches community taps. Chlorine residuals in drinking water are regulated by the EPA, and minimum requirements will vary depending on the state. Generally, a residual between .5 mg/L and 5.0 mg/L is safe for consumption2.
In addition to the breakpoint strategy covered in the wastewater section, drinking water plants also use chlorination strategies called marginal chlorination and super chlorination/dechlorination.
Marginal chlorination is the process of only adding an amount equal to what is the desired chlorine residual. It is used in applications where the source water has a low organic load, such as source water from an aquifer or other groundwater source. These water sources have a low chlorine demand, and thus marginal chlorination dosing will be sufficient. In many cases, breakpoint is never achieved because there is not enough organic material to react with the chlorine.

Water plant operator adjusting process
Super chlorination/dechlorination is the process of adding an excessive amount of chlorine to induce a rapid chlorination reaction and then performing a subsequent dechlorination process to drop to the desired chlorine residual. This process is often used in applications where the organic load is variable2.
For marginal chlorination and super chlorination/dichlorination strategies, it is vital to measure free chlorine. Drinking water municipalities must report their chlorine residuals at the plant's effluent. To report to the EPA, public water systems must measure residual disinfectant concentrations with one of the analytical methods outlined in the Code of Federal Regulations (40 CFR § 141.74). Free chlorine residuals are the main concern when it comes to effluent monitoring and reporting. Typically 0.2 mg/L Cl is the minimum required by the EPA, and 4.0 mg/L (running average) is the maximum.
For additional information and improved efficiency, total chlorine can be measured as well. Similar to wastewater process monitoring, measuring both total and free chlorine allows the operator to get a full picture of the free, total, and combined chlorines, which is particularly important if chloramines are critical to the disinfection process as they are considered a part of combined chlorine.

Drinking water from a tap
Distribution network monitoring is important to ensure that water quality is being maintained as it travels out to the community. Drinking water distribution systems may be accidentally contaminated through cross-connections with non-potable water, permeation of contaminated water through leaking pipes in areas of the distribution system subject to low pressures, or chemical reactions or microbial growth within the distribution system pipes3. Due to this, maintaining a free chlorine residual within the distribution network is extremely important.
As discussed earlier, free chlorine is the most reactive and effective disinfectant, so having a free chlorine residual available to neutralize any contamination during the distribution of potable water is key to safeguarding public health. Some municipalities will dose chloramines rather than chlorine throughout their distribution network. In this case, a total chlorine measurement combined with a free chlorine measurement will provide the most accurate data on the residuals available to neutralize any contaminants entering the distribution network.
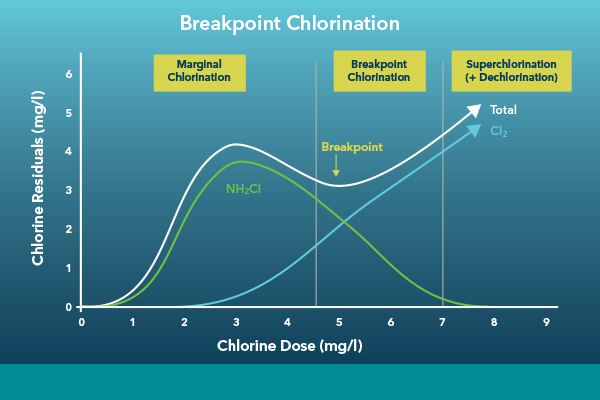
Breakpoint chlorination in drinking water treatment. Adapted from: Lian TadBir https://liantadbir.com/en/process/chlorination/
Although chlorination processes are highly effective in neutralizing waterborne pathogens, there are some downsides. Water that is over-chlorinated (> 5 mg/L) can pose potential health risks to humans and animals. Furthermore, the formation of disinfection byproducts is a major concern in the drinking water process. However, the risks associated with DBPs are much less when compared to the risk of waterborne pathogens that are found in unchlorinated water. There is a balancing act that takes place when water plants use chlorine-based disinfectants. Because of this, a sound chlorine control strategy is a key factor in safeguarding public health.
Additionally, chlorine chemicals are expensive to buy and store. By developing a sound chlorine disinfection strategy, drinking water plant operators can ensure public health and safety while also eliminating the potential of wasting chemicals by unnecessarily dosing the water.
What Instrumentation Can Be Used to Measure Free and Total Chlorine?
Chlorine analyzers that use a colorimetric analysis method called the N, N-diethyl-p-phenylenediamine (DPD) method have been the gold standard for measuring free and total chlorine. The DPD method is an accurate and reliable measurement that has been used since the late 1950s for free and total chlorine measurement. This is a reagent-based method that reacts with chlorine in a sample turning the solution to a magenta color.
YSI’s 3017M DPD Chlorine Analyzer uses two reagents, a buffer and a reactive reagent containing the DPD compound. The buffer solution helps ensure that the pH of the sample is between 7 and 8. The reactive reagent contains DPD and this is what reacts with free chlorine. Potassium Iodide (KI) is added to the DPD reagent when measuring total chlorine. KI reacts with chloramines, and the DPD reacts with free chlorine, resulting in the measurement of total chlorine.
The 3017M uses Flow Injection Analysis (FIA) to thoroughly mix the reagents and sample. FIA is based on the injection of a liquid sample into a moving, nonsegmented continuous carrier stream of a suitable liquid. In the case of the 3017M the process sample is the carrier stream, and the buffer and DPD reagent are what is being injected. The injected sample forms a zone, which is then transported toward a detector that continuously records the changes in absorbance, electrode potential, or other physical parameters resulting from the passage of the sample material through the flow cell4.
FIA limits the number of moving parts in the analyzer, which helps safeguard it from mechanical failures that other mixing methods are prone to. The 3017M has a continuously running sample pump that helps to keep the analyzer tubing clean for longer periods in clean water applications. This constant flushing of the system helps to prevent DPD staining, which can occur in instrumentation that does not adequately remove DPD reagents between measurements.

Online analyzers can be used to continuously measure free and total chlorine.
Another analytical method commonly used to measure chlorine is the amperometric method of analysis. This method of determining chlorine uses an electrochemical technology where two dissimilar electrodes change in current based on the amount of chlorine in a given sample. The MAperometric method of analysis can be packaged in an insitu probe or in a side stream analyzer. Arguably the greatest differentiator an main benefit of this method is that it doesn’t require reagents to determine the chlorine concentration. However, the amperometric method is dependent on consistent pH, sample temperature, flow, and pressure. For more information on how the DPD and amperometric methods stack up against one another check out this infographic: DPD (Colorimetric) vs. Amperometric (Electrode) Method
Conclusion
Both free chlorine and total chlorine levels are important parameters to measure in water treatment and sanitation. Free chlorine is the most important parameter to measure when ensuring water is safe for human consumption, while total chlorine is important to measure when monitoring the effectiveness of chlorine disinfection and its impact on the environment. In general, if the combined chlorine level is important to process control, monitoring, or reporting, both free and total chlorine should be measured.

References
- Center for Disease Control and Prevention, Water Disinfection with Chlorine and Chloramine
- World Health Organization, Guidelines for Drinking-Water Quality, 4th Edition
- United States, Congress, Office of Water. Interim Guidance on Planning for Contamination Warning System Deployment, U.S. Environmental Protection Agency
- Encyclopedia of Analytical Science (Second Edition), FLOW INJECTION ANALYSIS | Detection Techniques