Dissolved Oxygen Measurement Frequently Asked Questions (FAQs)
(Updated: August, 2021)
Whether you're interested in buying a dissolved oxygen meter or are just curious about how a dissolved oxygen meter works, we have some answers for you. Based on some frequently asked questions we've put together this list on DO calibration, cleaning, maintenance, and how to get the best measurements.
How often should I calibrate my dissolved oxygen meter?
As a rule, YSI recommends that a dissolved oxygen meter calibration be performed or verified daily before sampling starts. But in general, calibration frequency is determined by the user and the importance of the data. The more critical the data, i.e. when used for compliance purposes, the more attention should be paid to timely calibrations.
The calibration of optical-based dissolved oxygen meters is very stable but YSI still recommends that it be verified on a regular basis to ensure accurate data. Your data is as good as your calibration.
So, short answer, once daily but it's worth verifying the calibration occasionally throughout your sampling.
How do I verify my dissolved oxygen meter calibration?
Place the sensor in its calibration environment and check to see that the instrument is reading the calibration value for the current barometric pressure. For example, if your ‘true’ barometric pressure is 750, divide this number by 760 and then multiple by 100% to calculate what your instrument should be reading in water-saturated air or air-saturated water.
750/760 x 100 = 98%
A post-check can also be performed with this value in mind. If, for instance, you calibrate your instrument to 98% and then conduct your DO testing, you can place the sensor back into the same calibration environment and it should read +/- 2% (+/- 1% on optical) of 98% once stable.
You must look at the % value instead of the mg/L value (which is a calculated number).
Sampling scenario:
If you're on a long sampling trip and you calibrate in the morning before heading out and it calibrates to, let's say 98% again as we reference above, and you conduct some sampling then power the meter off will this have an effect? This is where verifying comes into play as well. You've now headed to your next site and you power the DO meter back up and let it equilibrate with the probe in the cal chamber. Does the reading come back to 98% or within the margin of accuracy range? If so, get on out there and measure your DO. If not, there could be other issues and/or you may need to calibrate again.
What is ‘true’ barometric pressure?
DO instruments need to be calibrated to “True” barometric pressure, i.e. a barometric pressure value that has not been corrected to sea level. Laboratory barometer readings are usually “true” (uncorrected) values of air pressure and can be used “as is” for DO calibration.
Weather service readings are usually not “true”, i.e., they are corrected to sea level, and therefore cannot be used until they are “uncorrected”. An approximate formula for this “uncorrection” is:
True BP = [Corrected BP] – [2.5 * (Local Altitude in ft. above sea level/100)]
Does the calibration environment affect my readings?
In short. Oh yeah. If you have an environment that the DO sensor is stored in and used to conduct the calibrations and it is 'dirty' it could definitely cause an oxygen-consuming (deficient) environment and ultimately affect your DO values. If you're conducting BOD readings make sure the BOD bottle that the probe is stored in is kept clean. Similarly, for field DO sensors, if you use a sponge, paper towel, etc. to keep the environment saturated make sure it stays clean!

An example of a clean sponge (left) and fouled paper towel (right). This paper towel was being used to house a DO probe in the calibration chamber. The readings were not consistent and it was due to this fairly nasty calibration environment.

The BOD bottle on the left is an oxygen-consuming environment while the bottle on the right is a clean calibration environment. Switching these probes from bottle to bottle showed consistent low DO readings in the 'dirty' bottle.
If a calibrated DO instrument is turned off, then back on, does it need to be recalibrated?
There is no need to recalibrate new digital or older analog models when the instrument is turned back on. Instruments are designed to record and save the calibration values so no recalibration is required at power-up unless it requires normal, daily routine calibration.
You can also “verify” that the instrument is holding its calibration by knowing the original calibration value (98% as an example) and the instrument should read within +/-2% (+/- 1% on optical) of 98% upon power on once it’s stable as we demonstrated above.
After turning the instrument on, how long should I wait before calibrating or taking a measurement?
There really is no set time period. Regardless of the sensor type that is used, wait for the temperature and dissolved oxygen values to become stable. For dissolved oxygen systems that use a polarographic sensor, achieving stability takes about 5-15 minutes. For galvanic and optical sensors, the readings will reach stability almost immediately after turning the instrument on unless the ambient temperature has changed. For each sensor type, it is recommended to make sure there are no water droplets on the sensing element (membrane) to help ensure quicker stability and ensure calibration accuracy.
For the best calibration results, it is very important to have the DO system in an environment where the temperature is stable and does not change prior to, or during the calibration procedure.
It's also important on instruments that have screw-on calibration cups to unscrew the cup just a thread or two in order to ensure the pressure in the cup and outside the cup is the same and to allow the temperature to equilibrate.
Is it necessary to recalibrate if there is a change in altitude or barometric pressure after the initial calibration?
No. Dissolved oxygen sensors are calibrated to and measure the partial pressure of oxygen. Therefore, after performing an accurate calibration, the sensor will automatically compensate for changes in pressure. For systems that are using the DO% Local function, the calibration value will be 100% regardless of the altitude or barometric pressure and the internal barometer will be used to keep the saturated value at 100%.

For consumptive electrochemical sensors, how do I know when I have enough stirring?
When increasing the amount of stirring does not result in an increase in the dissolved oxygen readings, enough stirring is being supplied.
Say you simply set the sensor in a stream with a languid flow and allow the reading to stabilize and it reads, for instance, 6.15 mg/L and you then provide some stirring to the probe and the reading goes to 6.87 mg/L, there wasn't sufficient stirring initially with the stream flow itself. If you then stir even more and the reading stays at the 6.87 range then no more stirring is necessary.
How long does it take to get my dissolved oxygen reading?
The response time of a sensor should be considered when selecting an instrument since this will dictate the amount of time required to conduct a sampling session and the number of samples required. Although 40 seconds is a relatively short period of time to take one reading that same response time over 500 samples would require 5 hours.
See the response times below based on T-95 which is the length of time it takes to reach 95% of the final (true) reading when a sensor is transferred from a fully saturated sample to a zero oxygen environment.
Measurement Response Times of Sensors and Membranes
| Sensor Type |
Membrane Material |
Response Time 100 to 0% (T-95) |
| Optical |
Sensing element with diffusion layer |
40 seconds* |
| Galvanic or Polarographic |
1.0 mil Polytetrafluoroethylene (Black cap membrane and stretch-over style) |
18 seconds |
| Galvanic or Polarographic |
1.25 mil PE (Yellow cap membrane) |
8 seconds |
| Galvanic or Polarographic |
2.0 mil PE (Blue cap membrane) |
17 seconds |
* YSI studies have shown that stirring an optical sensor sample can lower response time. For example, using a magnetic stirrer or stir bar could result in an optical response time of 22 seconds or less for T-95.
Does a turbulent application and/or bubbles affect my DO reading?
It's very likely that a turbulent application or bubbles rising and bursting on a membrane could affect the reading. More than likely, the reading would be bouncing around as the membrane shape changes or bubbles burst on, or settle on, the membrane itself. Optical sensors are less likely to encounter these problems.
One way to deal with turbulent waters is to simply orient the sensor at a 90 degrees angle to the flow of the water. Simply make sure the tip of the sensor isn't pointing into the turbulence.
If there are bubbles rising up through the water then you may need to invert the instrument (sonde) or the probe on a handheld instrument.

In order to avoid 'jumpy' readings from bubbles, the DO probe can be inverted and attached to its own cable. Ensure there is a gentle loop in the cable vs. a hard crimp.
How often should the membrane be changed on an electrochemical sensor?
As a general rule, YSI recommends that the membrane be changed every 2-8 weeks. This is dependent on the sampling application and frequency of use. Additionally, keeping the membrane clean and in a moist environment between uses will lengthen the membrane life.
When measuring samples with high levels of hydrogen sulfide, a weekly membrane change will reduce sensor cleaning and maintenance and thus provide better performance. When measuring in clean water, membrane changes can easily go beyond 4 weeks without negative effects.
>> See how to change the stretch-over style membrane, DO cap membrane or EXO optical DO sensor cap.
How often do electrochemical sensors need to be cleaned or serviced?
Maintaining electrochemical sensors is relatively easy but is only necessary when the sensor no longer performs to factory-established specifications.
During calibration, YSI instruments perform a sensor performance check. If the sensor is outside of its normal working parameters, the instrument will give an out-of-range indication. Before cleaning electrodes, always try fresh probe electrolyte solution and a new membrane.
It is normal for a polarographic sensor’s silver anode to darken over time due to the build-up of silver chloride. This typical darkening will not affect the performance of the sensor so do not clean the electrodes just because they ‘look dirty’. Long-term exposure to hydrogen sulfide can cause darkening of the anode that will influence a sensor's performance. Typical symptoms will be jumpy readings, inability to calibrate and/or low probe current, both of which may respond well to cleaning. Typically, the electrodes will require cleaning or servicing about once per year.
Go to the troubleshooting and maintenance section of the instruments instruction manual (ProSeries instrument onboard “Help”) for the best advice regarding resolving these issues.
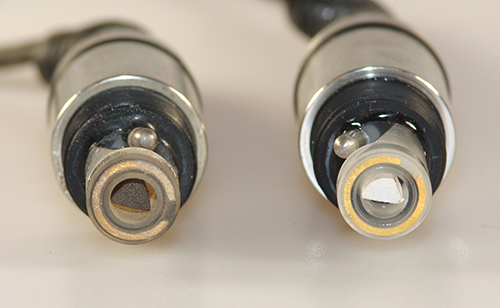
The DO probe on the left shows an anode that has darkened over time and use. (The black triangle piece). This darkening in and of itself isn't cause for concern but if the sensor won't calibrate or hold its cal value after changing the DO membrane and electrolyte solution, it may be time to service the anode and cathode (gold ring around the tip).
How often should I replace an Optical sensing element?
The optical sensing elements are warranted for 1 year but experience shows they may last longer. Be sure to keep the sensing element clean and stored in a moist environment between uses to obtain the longest usable life possible. Perform calibration checks as mentioned above in 'How do I verify my calibration' to verify the sensor stability.
>>> If you have additional questions to add to this list, let us know and we'll update this post as needed.

We Know DO. Visit the dissolved oxygen page to see more resources!
Additional Blog Posts of Interest
Nonload Parameters in Surface Water | Dissolved Oxygen
Environmental Dissolved Oxygen Values Above 100% Air Saturation
5 Easy Steps: Replacing the Optical DO Sensor Cap on YSI EXO Sondes
A Step-by-Step Guide to Installing Stretch-Over DO Membranes