YSI’s customers are occasionally concerned about observing “Percent Air Saturation” dissolved oxygen readings in environmental water (lakes, streams, estuaries, etc.) that are above 100%. The issue is usually one of semantics. How can something be more than 100% saturated? To understand the overall concept, it is necessary to consider the sources of dissolved oxygen in environmental water and to appreciate that equilibration between air and water is rarely perfect in environmental situations. Air is certainly one source of dissolved oxygen in environmental water. If air were the only source of oxygen and if environmental water equilibrated with the air above it instantly during temperature changes, then it would indeed be impossible to observe values above 100% air saturation unless the sensor was in error. Neither of these “if statements” is true, however, for most bodies of water.
Oxygen Sources
Photosynthetically-active species (plants, algae, etc.) are common additional sources of dissolved oxygen in the environment and, in many bodies of water, can, in fact, be the dominant factor in determining the dissolved oxygen content. It is important to remember that these organisms produce pure oxygen (not air) during photosynthesis. Air is approximately 21% oxygen and thus it contains about five times less oxygen than the pure gaseous element produced during photosynthesis. The oxygen content of any liquid is defined by Henry’s Law as being proportional to the partial pressure (or percent) of oxygen in the gas above it.
In practical terms, this means that if air and oxygen from compressed gas cylinders are bubbled into separate water samples, the sensor reading from the oxygen-saturated water will be about five times larger than that of the sensor reading from the air-saturated water. If the sensor is calibrated to 100% in air saturated water or water-saturated air (as is done for most YSI meters), then the reading in oxygen-saturated water will be about 500% air-saturation. There is no difference between the oxygen from the compressed gas cylinder in the above hypothetical experiment and that produced by photosynthetically active species in environmental water. Thus, photosynthesis can readily account for “percent air-saturation” values of between 100 and 500% depending on the efficiency and concentration of the photosynthetically-active species present.
Non-Ideal Air/Water Equilibration
Another possible cause of dissolved oxygen readings greater than 100% air saturation arises from the fact that equilibration (or equalization) of the oxygen content of water with the air above it is seldom rapid except in fast-flowing streams. This fact allows temperature changes to produce water conditions that lead to dissolved oxygen readings of over 100% air saturation. The following example may be useful in understanding this concept: The dissolved oxygen reading of a relatively stagnant lake at night is 9.65 mg/L when the temperature is 17°C. This corresponds to 100% air saturation. During the next day, the sun warms the water to 22°C where 8.22 mg/L represents the 100% air-saturated value.
However, the temperature change has occurred rapidly enough to prevent the oxygen in the water from “escaping” to the air because of non-ideal equilibration conditions. The lake still contains 9.65 mg/L of dissolved oxygen, but now the temperature is 22°C where 9.65 mg/L corresponds to 117% air-saturation ({9.65/8.22} x 100). If the lake had been equipped with an efficient aerator, the equilibration process would have been rapid and prevented the observation of readings greater than 100% during the temperature change.
Field Data
From our extensive experience in the field and testing our instruments at the YSI facility, values over 100% air saturation have indeed proven to be quite common. The documented studies in three separate bodies of water in southwest Ohio demonstrate where these “over-saturated” values have occurred.
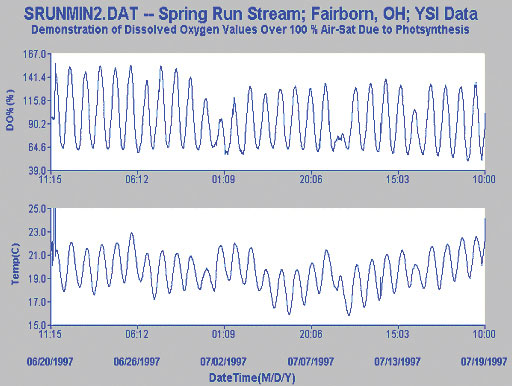
This first study is of a slow moving stream where a large diurnal cycle is observed for dissolved oxygen. This pattern is thought to be primarily due to the photosynthesis by day and respiration by night of the “green” organisms in the stream, although temperature changes with non-ideal equilibration between air and water may also contribute to the cycle.
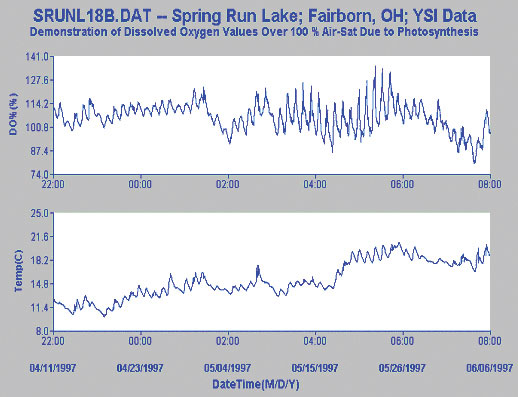
The second study pertains to a very clear, spring-fed 12-acre lake which contains a variety of weeds and suspended algae. Again, dissolved oxygen readings above 100% air saturation are common.
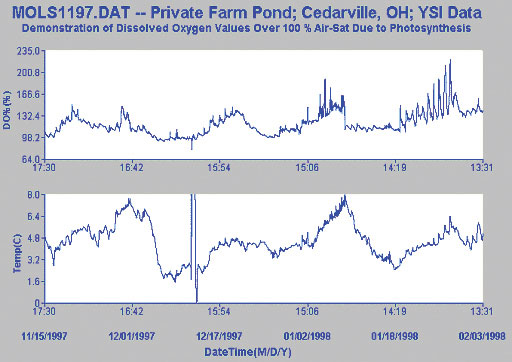
The third study shows data from a small farm pond that has a very high algal content. It can be seen from the data taken in this study that readings well over 200% air saturation were observed during the study.
When unusual or unexpected sensor readings are encountered in environmental water, it is important to have post-study quality assurance data to prove that the sensor was functioning properly. Standard YSI QA forms are provided with the data plots to show that the sensors did not drift significantly during the studies and that the DO data are reliable. Note that the DO drifts (as determined by placing the sensor in air-saturated water or water-saturated air after the study) were -5%, -2%, and +1.8 % for the three studies, respectively.
Summary
Dissolved oxygen readings of greater than 100% air saturation can occur in environmental water because of the production of pure oxygen by photosynthetically-active organisms and/or because of non-ideal equilibration of dissolved oxygen between the water and the air above it. In YSI’s experience, this “over saturation” is quite common, with photosynthesis being the factor most often responsible for its existence.
While it is clear that readings greater than 100% air saturation are possible in environmental water, only a well-defined standardized quality assurance program will provide confirmation that the over saturated readings are correct and not due to calibration error or sensor malfunction.
Additional Blog Posts of Interest:
What is Affecting Your Dissolved Oxygen Measurements? Part 1 of 4
What is Affecting Your Dissolved Oxygen Measurements? Part 2 of 4
What is Affecting Your Dissolved Oxygen Measurements? Part 3 of 4
What is Affecting Your Dissolved Oxygen Measurements? Part 4 of 4