UV Sensors Help Shortcut the Nitrogen Removal Process
Nitrite, long disregarded as an insignificant intermediate in the wastewater nitrification process, has recently gained prominence. Research and practice have identified nitrite as the critical variable in newly-developed treatment processes that utilize shortcuts in the nitrogen cycle for more efficient nitrogen removal from wastewater*.
The Traditional Process for Nitrification/Denitrification Proceeds as Follows:
1) ammonia (NH3) is oxidized to nitrite (NO2) and nitrite to nitrate (NO3) with the input of oxygen
2) nitrate is reduced to nitrite and nitrite to nitrogen gas (N2) which escapes to the atmosphere with the input of organic carbon and excluding oxygen (Figure 1).
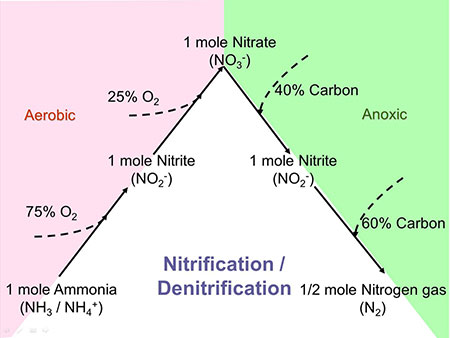
Figure 1
The process requires the input of 4.6 lbs. of oxygen and 3.7 lbs. of chemical oxygen demand (COD) and consumes 3.6 lbs. of alkalinity for every lb. of nitrogen removed.
This requires a substantial input of energy to drive the blowers that dissolve oxygen into the reactor and to power the pumps required to recirculate nitrified biomass to the head of the bioreactor to utilize wastewater COD.
It may also require the input of an external source of carbon, for example, methanol or MicroC™, if total nitrogen concentrations less than 10 mg/L are to be achieved. Monitoring of nitrate is critical for the control of sludge recirculation and external carbon dosing. Furthermore, monitoring of nitrite is critical to avoid an upset condition called “nitrite lock”, an undesirable accumulation of nitrite, which causes a substantial increase in chlorine demand for effluent disinfection.
Nitrification and denitrification both proceed through nitrite suggesting a possible way to reduce oxygen and carbon requirements. However, the conventional wisdom was that oxidation of nitrite was rapid and uncontrollable. This was based, in part, on the inability to efficiently and reliably measure nitrite continuously and in real-time. In nitritation/denitritation the nitrogen pathway and organisms involved are identical to traditional nitrification/denitrification except that nitrate is cut off (Figure 2).
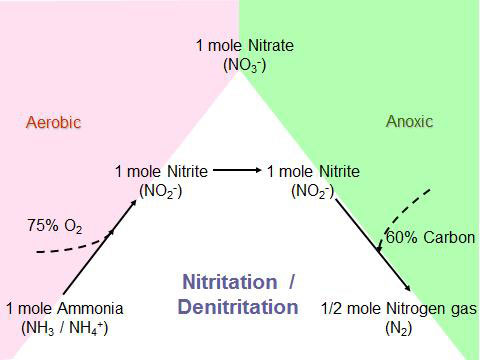
Figure 2
Multiple processes have been developed based on inhibiting nitrite oxidation. The nitrite which accumulates is reduced to nitrogen gas. This process is termed nitritation/denitritation.
Figure 2 illustrates that nitritation/denitritation reduces the oxygen requirement by 25% and the carbon requirement by 40% compared to the conventional process. The Single reactor system for High activity Ammonium Removal Over Nitrite (SHARON) process developed in the late 1990s exploits the condition that ammonia oxidation proceeds faster than nitrite oxidation above 30°C. The additional requirement for heat has limited application of SHARON to the treatment of nutrient-rich recycle streams from dewatering of anaerobically-digested solids. Monitoring of nitrite and nitrate is critical for control of ammonium oxidation to nitrite and preventing conditions resulting in nitrite oxidation to nitrate.
Deammonification utilizes a totally different pathway to oxidize ammonia directly to nitrogen gas anaerobically (anammox reaction). The first step is partial nitritation of half of the ammonia to nitrite. In the next step equal amounts, the ammonia and nitrite are oxidized to nitrogen gas (Figure 3).
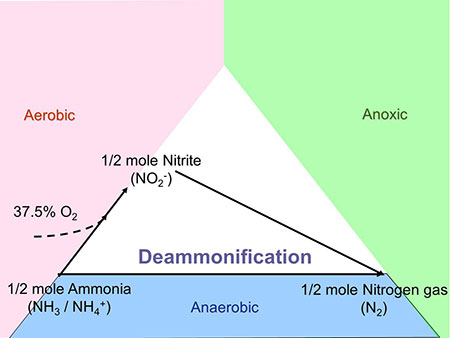
Figure 3
The benefit is that over 60% of the oxygen demand and 100% of the carbon demand is eliminated compared with conventional denitrification saving energy and chemical inputs. Deammonification also was originally implemented for the treatment of solids sidestreams. Additionally, deammonification is being intensively studied for mainstream implementation at wastewater utilities in the sensitive Chesapeake Bay watershed.
In the main-stream implementation, control of dissolved oxygen (DO) to low concentrations is the favored method for suppression of nitrite oxidation. Monitoring of nitrite is critical because excessive nitrite accumulation causes a severe process upset and must be avoided.
Nitrogen removal processes utilizing shortcuts in the nitrogen cycle offer tremendous potential to save energy and money. Infrequent grab sampling followed by manual laboratory analysis is not adequate to control the process. Online, immersible UV spectral sensors that have the ability to monitor both nitrate and nitrite with one instrument are key for controlling these processes. (Learn more, Top 5 Questions When Selecting UV or UV Vis Sensors.) Furthermore, no reagents are required and complicated sample processing systems are not needed using a system such as the IQ SensorNet.
* Melcer, H, et al. “Measuring Nitrite - The Key to Controlling Deammonification Systems”, Proceedings of the WEF/IWA Nutrient Removal and Recovery Conference, Vancouver, CA, 2013

Additional Blog Posts of Interest:
Can Smart Devices Enhance Water Resource Recovery Facilities? Part 1
Can Smart Devices Enhance Water Resource Recovery Facilities? Part 2
Wastewater Aeration Control - Energy Cost Savings Examples
Troubleshooting Nitrification and Denitrification in Wastewater