Tips for Karl Fischer Sample Preparation in Pharmaceuticals
Pharmaceutical products can come in all states of matter — solid, liquid, or gas. Each form of matter requires different sample preparation and handling procedures. The process requires precision, accuracy, and systemic monitoring to meet quality and safety expectations. Materials are added at a constant rate to ensure consistency. This blog will help determine what laboratory equipment is necessary for adequate sample preparation.

Each ingredient, and the amount of that ingredient, plays an essential role in the production process. Water content impacts many physical properties including weight, density, shelf-life, and stability, and there is a possibility each ingredient could contain water. The water content ensures proper crystallization, agglomeration, and chemical formation and production of solid tablets. Pharmaceutical products that are solid and compact may incorporate ingredients with skin irritants or inhalation hazards, making these physical properties extremely vital for safety.
Because it is essential to monitor the water content throughout the entire manufacturing process it is important to know what instruments are suited for the job. Karl Fischer, a titrimetric method, is one of the most common analysis used for water content measurements. And YSI Titration has a strong portfolio of instrumentation for your analysis.

Sample properties
The working methods of Karl Fischer titration are highly dependent on the properties of the sample. Working with gaseous, solid samples, low-viscosity, and high-viscosity liquids is distinguished by:
- Sample input
- Solubility
- Sample quantity
- Sample volume.
- Water yield
Working with Liquid Samples
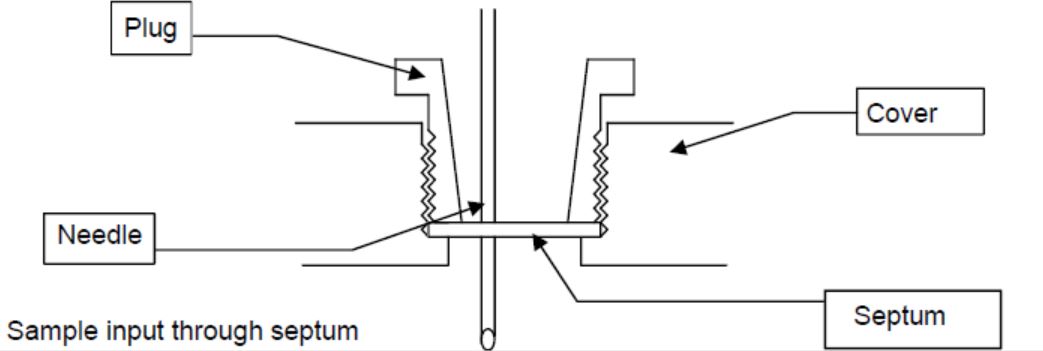
In general, liquid samples are injected through a septum into the titration cell using a one-way syringe. The size of the syringe depends on the sample volume and it is not uncommon for syringes to range from 1 mL to 20 mL.
The needles of these syringes have a diameter ranging from 0 .6 to 1.2 mm. Thinner needles are only available for low viscosity samples, whereas the thicker needles should be used for samples with a higher viscosity. The thicker the needle is, the higher will be the degree of the effect on the septum, which will cause it to be replaced sooner. The length of the needles should be between 50 and 90 mm, so it can be ensured that no drops remain adhered to the walls. Immersing the needle into the solvent should be avoided.
Particularly high-viscous samples, such as oils, are transferred into the titration cell without a needle and without the use of a septum.
The septum is made of a polymer that should be properly sized to fit the apparatus and should have close alignment directly after being pierced. These silicone discs should also be replaced on a regular basis and the frequency will depend on the amount of usage it incurs.
If you are working with liquid samples, check out this application note: Titration of Water in Liquid Samples
The Working Procedure
Using good laboratory practice, the samples have to be weighed using an analytical balance. The working procedure for liquid sample preparation is as follows:
- Put a 150 mL glass beaker on the balance
- Draw up the liquid sample into the syringe
- Put the needle protector on again
- Place the syringe with the needle up into the beaker glass
- Tare the weighing-balance
- Carefully take the syringe from the balance, then remove the needle protector
- Inject the sample through the septum into the titration vessel
- Remove the syringe from the septum, put on the needle protector again
- Place the syringe with the needle up in the beaker glass on the weighing-balance
- Read off the weight as a negative number on the weighing balance, then enter it as an absolute value on the titrator
When drawing up the sample into the syringe, please proceed carefully and slowly to avoid air being unnecessarily drawn through the samples, which may introduce airborne humidity to the sample.
Working with Solid Samples
There are two common methods for solid samples – direct sample input and the use of a solid lock.
Direct Sample Input
Direct sample input is certainly the simplest way of introducing solid samples in the Karl Fischer titration vessel. During the opening time of the cell, the humidity of the ambient air may lead to a blank value. Determine the blank value in a separate titration, and subtract the difference. The smaller the water quantity to be determined, the less favorable is this method of sample addition. As a rule of thumb, the blank value should not be greater than the water quantity to be determined.
Weigh samples with a glass or aluminum weight boat for easy transportation to the titration vessel. After weighing the samples, be sure they are no longer touched by hand.
Use of a Solid Lock
With the solid lock, it is possible to re-condition after the titration vessel was opened, prior to introducing the sample in the solvent. The sample quantity is limited to small sample quantities in the range of one gram.
The Working Procedure
The working procedure is based on the following scheme:
- The sample is weighed inside the solid lock.
- Pull the outer part of the solid lock over the inner part containing the sample, until the part containing the sample is fully closed.
- Open the titration vessel, then place the outer part of the solid lock with the built-in NS 19 ground surface in the opening.
- Start conditioning.
- Push down the inner part, so that the sample can be dissolved away.
- Start titration with a longer extraction time for solving the sample. As an alternative, a minimum titration duration can be specified.
Working with the solid lock may involve a higher drift. This can be taken into account accordingly in the titration methods
Dry-Heating Samples Using an Oven
In many cases, a direct determination is impossible because:
- The samples are insoluble (plastics).
- The sample releases the water upon heating.
- The sample enters into side reactions.
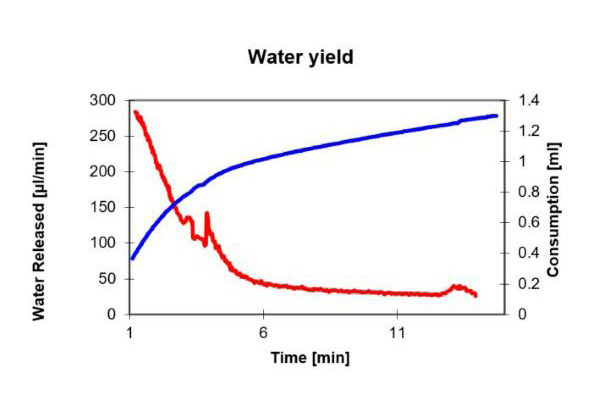
The samples are placed in the oven then heated and the water vapor is transferred to the titration vessel by dried gas. During this process, the majority of water is released at the beginning. The following graph shows how the water contained in the sample is released. At the end of the titration, the added quantity is limited by the drift value. On the graph, a side reaction is indicated by a high residual drift. The x-axis shows the duration of the titration, the first y-axis shows the drift, the second y-axis shows the consumption.
After approximately five minutes, the major part of the water is released. We recommend a dry-heating time of 10 minutes. This time is set as a waiting time on the titrator. Subsequently, the water quantity absorbed in the solvent is titrated within a short time frame. If the preceding drift is not reached again, or almost not reached again, this is indicative of a side reaction.
Read Blog Post: Top Tips for More Accurate Titrations
With the titration in the graph, it was proven that thermal decomposition leads to the generation of formaldehyde, which reacts with the methanol in the titration cell to form acetal, with water being separated.
YSI is proud to offer a full titration portfolio for liquid and solid samples. YSI titrators provide the highest quality instrumentation for manufacturing facilities. YSI TitroLine® 7500 KF has multiple method availability – sample titration, titer water, titer liquid blank value oven and more.

Any widely used titration parameters are incorporated into this unit, including the option of customization. When titration requires advanced technology or versatility, YSI has a solution.
