How Do Small pH Changes Affect Ocean Acidification
(Updated October 2021)
The negative effects of climate change caused by greenhouse gases are familiar, however many people don't understand the impacts of ocean acidification, which is often called the “evil twin” of climate change.1 Awareness among scientists and the public is relatively recent, but this issue has the potential to become one of the most pressing and dangerous consequences of fossil fuel emissions.

Image 1. Carbon dioxide is emitted through burning fossil fuels.
Ocean acidification is essentially the rapid reduction of pH in seawater caused by the ocean’s absorption of carbon dioxide (CO2) emissions.2 CO2 occurs naturally in the atmosphere but is also a manmade greenhouse gas that’s produced by burning fossil fuels like coal and natural gas from factories and transportation.
The Prediction
Ocean acidification began in the industrial era in the 18th century and is quickly accelerating. Before the Industrial Revolution, the average pH of surface seawater was 8.2 and the current surface seawater pH is about 8.1.3
Only a 0.1 drop may not seem concerning, however, the pH scale is logarithmic with each increment actually representing a tenfold change. (Learn more, Why is the pH Scale Logarithmic?). Therefore, this 0.1 drop represents a 25% increase in acidity.3
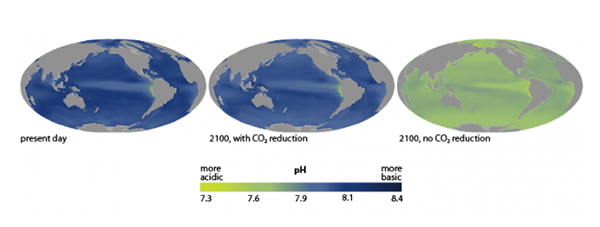
Image 2. The predicted change in ocean pH by NOAA4
According to the National Oceanic and Atmospheric Administration (NOAA), if we don’t take any CO2 emission-limiting steps, our ocean’s surface is predicted to drop to a pH level of 7.67 by the year 2100.4 Currently, the pH of the ocean’s surface is about 8.1. This drastic 0.43 drop has never occurred in approximately 21 million years and it has the potential to become a big problem; scientists are unsure if ocean life is able to adapt that quickly to the change in acidity.4
Putting the predicted 0.43 drop in pH into perspective, it could result in a 165% increase in surface ocean acidity.
Let’s Break It Down
Scientists estimate that one-third of the harmful CO2 released into the air from burning fossil fuels is directly absorbed by seawater (about 22 million tons of CO2 a day).4 So how can CO2 absorption increase the ocean’s acidity to a near-devastating level?
First, the CO2 reacts with H2O and creates carbonic acid:
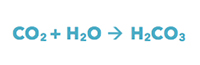
The carbonic acid releases hydrogen ions and becomes bicarbonate:

Then, the bicarbonate releases more hydrogen ions and becomes carbonate:
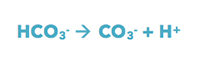
This process releases H+ into the water with each step and decreases the pH of the seawater. (Learn more, Is pH the Measurement of Hydrogen Ion Concentration or Ion Activity?).

Biological Impact
Much like other rapid climate changes, seawater acidification will drastically change the ecosystem and affect ocean food chains, available resources, habitats, and many natural processes of marine life if they fail to adapt in time.
With the ocean’s chemistry changing, calcium carbonate is being affected in a major way; it becomes more soluble or dissolved, in acidic water.5 Calcium carbonate (CaCO3) is a chemical compound used by both plants and animals in the mineral forms of calcite and aragonite.6

Image 3. Mussels growing on a rock on the coast
Most notably, shellfish can use both calcite and aragonite layers to build their protective and beautiful coverings, known to us as seashells.6 In acidic seawater where calcium carbonate is less present, the water becomes corrosive to the structures they build. This makes it increasingly difficult for vulnerable shellfish larva to develop their shells.7 Older shells can also become eroded, thin and fragile and thus less protective. Shellfish, specifically mollusks like clams, mussels, oysters, snails and scallops, are the foundation of the ocean food chain and endangering them endangers the entire marine ecosystem.7

Image 4. Sea Fan coral thriving in the ocean
The decrease in calcium carbonate in seawater also poses a threat to coral reefs. A coral plant needs enough aragonite, a mineral form of calcium carbonate, in the water around it in order to build its skeleton.8 It constructs a framework of aragonite crystals to grow toward the sunlight.9
Today, around 60% of all coral reefs are surrounded by water without an adequate amount of aragonite saturation. This is a major decrease from the 98% recorded in the pre-industrial era.9 As the pH of the water rapidly changes the corals can’t extract enough aragonite resulting in fragility and failure to develop.10

Image 5. Snorkeler by coral reef in the Red Sea, Egypt
If these shellfish and corals fail to adapt, these seemingly small consequences will turn into huge ones for the entire ocean’s ecosystem. Coral reefs are often used as a nursery for young fish and a home for many sea creatures that could disappear with the coral. Similarly, if shellfish dissolved away the marine lives that depend on them would disappear and drastically lower biodiversity.
Human Impact
Back on land, humans are inherently and vitally connected to the health of the ocean, prompting us to protect its ecosystem.
Because of the currents, shellfish farms on the Pacific Northwest Coast of the U.S. have already been hit hard by acidic waters. “We're the first to see the effects and to scream about it,” says Bill Dewey of Taylor Shellfish Farms, the largest producer of farmed shellfish in the U.S.7 Washington state’s shellfish industry alone is worth $270 million a year and responsible for thousands of jobs.7

Image 6. Oyster farming
Ocean acidification has socioeconomic impacts as well. Because fishing is a way of life for so many, demographics can change if working families move away in search of in-demand jobs.7
Just take a look at the potential devastation waiting in the Gulf of Mexico. The gulf reaches the shores of Alabama, Florida, Louisiana, Mississippi and Texas and generates over $10 billion in the fishing industry each year providing thousands of jobs to Americans. A decrease in marine life populations could result in a loss of seafood supply and those jobs over time.11
Finding Solutions
Unless carbon dioxide emissions decrease, ocean acidification is predicted to make an unwelcomed appearance in the near future and marine plants and animals will struggle to keep up. These negative impacts on specific organisms, like shellfish and coral, have the potential to ripple into larger consequences for the entire ocean ecosystem and humans.

Image 7. Scientist testing seawater with an EXO2 sonde
Not only should we be taking preemptive actions to significantly reduce our CO2 emissions, but we should also be tracking current conditions as well. As a relatively recent issue, intense research is crucial to finding solutions. Monitoring surface water using pH sensors is an important step in the process. As NOAA states, “We can’t manage what we don’t measure.”12 Because of vigilant monitoring in the past, this issue was discovered, studied and spread so we can now devise solutions before it’s too late.
At Xylem, we’ve been creating state-of-the-art technology to help environmental scientists accurately test and monitor the ocean for years. In a 2015 competition, Xylem developed an innovative pH sensor to be used specifically for ocean acidification monitoring. (Learn more, Improving pH to Monitor Climate Change and Ocean Acidifcation).
