pH Curriculum for Teachers
Our hats go off to all teachers, worldwide. Molding the minds of our youth is a tough job and we want to try to help make it a little easier and to show our love on this day. YSI has designed a Science curriculum for secondary education students (Grades 6-8) to help introduce them to pH and determining pH values!
This curriculum uses the YSI Ecosense pH10A pH pen-style tester, and has been designed to not only educate students but for them to have some fun in the classroom as well!
Objective:
- Introducing the concept of pH and the definition of pH – “A measure of the acidity or alkalinity of a solution, numerically equal to 7 for neutral solutions, increasing above 7 with increasing alkalinity and decreasing below 7 with increasing acidity. The pH scale commonly in use ranges from 0 to 14.” Certain chemical compounds release H+ (hydrogen ions) when they are in water and others release OH– (hydroxide ions). The pH of a solution is the measurement of the H+ or OH– present in solution. Acids release H+ in water and bases release OH– in water. The normal pH scale ranges from 0-14 and was designed to measure the acid or base content present in common chemical solutions. (Learn more, Is pH the Measurement of Hydrogen Ion Concentration or Ion Activity?).
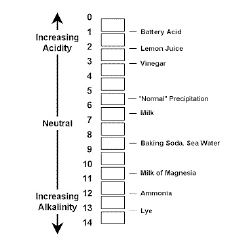
- Introducing the pH scale of 0-14 and how a solution is placed on this scale according to its pH value – students can also draw their own pH scales to help understand how to plot known values.
- Introducing the idea of a hypothesis. Students will “guess” what they think the pH value may be of a known liquid. Younger students can guess whether they think it’s more acidic or less acidic – older students can try to hypothesize what the range may be.
(Learn more, Why is the pH Scale Logarithmic?).
Materials:
- Known liquids they could make a hypothesis about: examples include, milk, tap water, Coca Cola®, lemon juice, pineapple juice, orange juice, etc.
- Known liquids that they are required to be CAREFUL with and DO NOT taste: examples include vinegar, soapy water, liquid drain cleaner, bleach, Milk of Magnesia, baking soda water, etc. Note – use proper caution when handling some of the above solutions, not appropriate for younger students to handle, and remind them to NEVER taste a solution to estimate its pH.
- EcoSense pH10A or pH100A instrument.

- Calibration solution for the instrument.
- Containers for liquids if not in appropriate sized containers.
- Paper towels.
- Safety glasses or gloves for older students allowed to handle non-consumable liquids along with proper ventilation if using bleach.
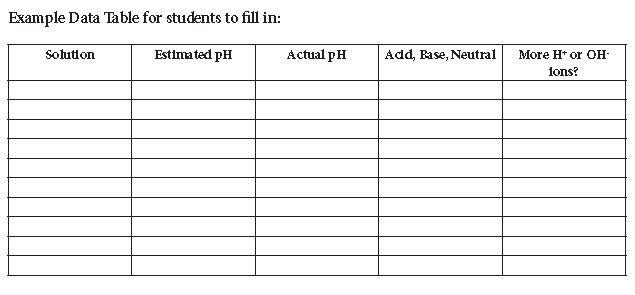
- pH Data Table to record values.
- Clean water or sink to rinse pH instrument electrode.
Procedure:
- Calibrate the pH instrument with the calibration solutions (pH buffers 4, 7, and 10). The teacher can complete this process easily or the students can assist. This allows students to use a scientific instrument and understand the process of acquiring scientific data. Students can also calibrate and record the calibration values for each buffer.
- Pick a liquid and discuss it briefly. What assumptions can students make about its acidity level based on taste or known uses of the solution? Have them make a guess and write it on their Data Table. Let them know that 7 on the pH scale is neutral and values below 7 are acidic while values above 7 are basic (not acidic).
- Complete the above step for all liquids.
- Once the students have placed their guesses on their data table start measuring each liquid and be sure to rinse and shake the electrode between solutions. Have them record the actual values on their Data Table next to their guesses.
- Next to each value on their Data Table have them determine if the solution is acidic, basic, or neutral. Students can determine if the solution is “slightly”, “moderately”, or “very” acidic or basic. Have the students determine if their definition means the solution has more Hydrogen (H+) or Hydroxide (OH-) ions. Remember, acid solutions have more hydrogen ions and basic solutions with more hydroxide ions.
- After testing all solutions, place the pH instrument electrode back into the pH 4, 7, and 10 buffer solutions as part of a post-calibration check. Record the numbers. See how closely the pH values of the post-check come to the recorded values of the calibration. This teaches students to check their data and understand how accurately the values of their tests are.
Additional:
- Have the students make hypotheses about what will happen if two substances of varying pHs are mixed together?
- Does the pH just average out in a 50/50 mixture or does it stay closer to the more acidic value? This is OK for students to do with consumable liquids – if older students are doing this with non-consumable liquids ALWAYS slowly pour the more acidic solution into the less acidic solution to prevent splashing of the acidic solution.
- Have the students add tap water to a solution to see how it affects the overall pH since it will be relatively neutral.
Have students make a pH scale similar to the one shown with the solutions used:
Thought Provokers:
- How accurate were the estimations?
- Can you closely determine the pH of a solution? Note: Remind students to NEVER taste a solution to try to determine its pH value.
- Which substance is the strongest acid, base?
- If a substance has a pH of 3 which of your solutions would you use to bring the pH up to 7?
- How do you think different pH ranges could affect fish or other aquatic life?
- Do you think different pH values could be dangerous to people? How?
- Do you think the pH in your stomach is acidic or basic? Why? (Stomach pH is approximately 2 to help break down and digest food).
Let us know how it goes, we would love to hear from you or your students!
Additional Blog Posts of Interest:
pH Electrode Reference Systems - A Brief Primer Why You Need It
Why Is the pH Scale Logarithmic?
pH Measurement Methods - Advantages and Disadvantages
Extend the Life of Your pH Electrode in 3 Practical Steps