Top 5 Tips for Total Cyanide Analysis

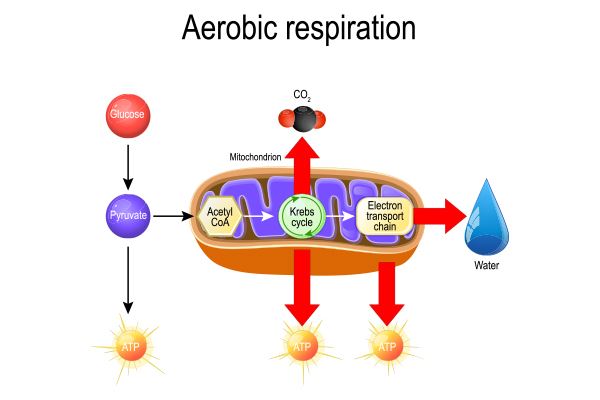
Highly toxic to animals and humans alike, cyanide (CN) pollution arises from a variety of industrial processes. Cyanide inhibits mitochondrial cytochrome oxidase, which blocks electron transport, resulting in decreased oxidative metabolism and oxygen utilization at the cellular level.
This results in aerobic cellular suffocation.
CN is produced in nature by certain bacteria and is also found in numerous foods and plants. Cyanide polymers are sometimes used in adhesives such as superglue. Hydrogen cyanide is a product of combustion found in automobile exhaust, cigarette smoke, and the burning of plastics.
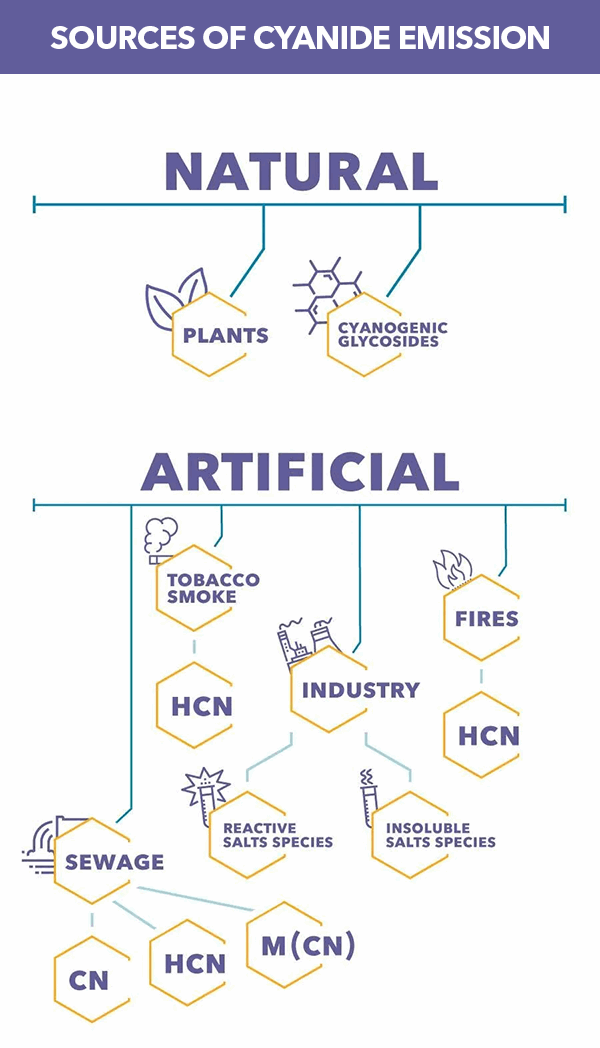
Cyanide is currently used in a variety of industries such as electroplating, pharmaceutical, metallurgy, photography, and precious metal extraction. These industries use various forms of cyanide complexes/solutions in their processes and produce cyanide-containing waste streams.
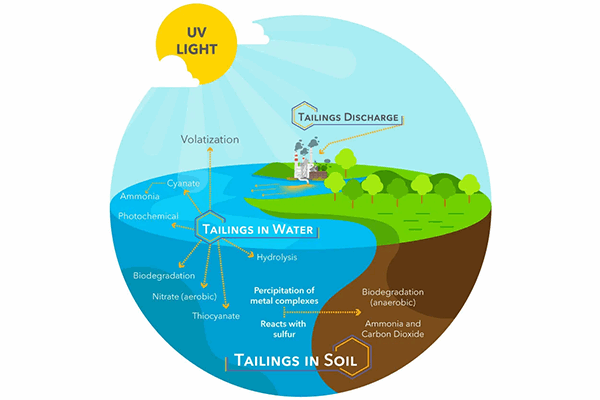
Cyanide releases from industrial processes in a variety of ways: Tailings from mining operations are one such example.
CN forms stable complexes with metals, and total CN in water is the combination of free, unbound, and metal-bound CN. These various forms affect CN measurements. Three types of CN analyses are typically performed in a laboratory:
- Total Cyanide
- Available Cyanide, or Weak Acid Dissociable (WAD) Cyanide
- Free Cyanide

Forms and Species of Cyanide
The five tips listed in this blog are for lab analysts who measure cyanide via segmented flow analysis (SFA) and flow injection analysis (FIA).
OI Analytical’s FS3700 is capable of measuring all three types of CN analyses utilizing the combination of SFA and FIA techniques.


The first tip is to select the right method: amperometric or colorimetric.
After you know which type of cyanide you're looking for, the next step is to select the proper detection method. The amperometric method reduces analysis time by eliminating the two-hour distillation step required for the colorimetric technique. This also minimizes interferences.
The ASTM D7511 Total Cyanide (amperometric) Method eliminates toxic reagents that generate laboratory waste and are unpleasant to work with. Rather than distilling, this method irradiates the sample with UV light at 312 nm. This irradiation effectively separates the cyanide from metal complexes that bind to it. The remaining cyanide is measured by gas diffusion amperometry.
For these reasons, the amperometric technique has become more prevalent among labs required to monitor cyanide concentrations in wastewater, drinking water, and industrial wastewater.

In principle, chemical reactions determine the curve fitting that should be chosen for a calibration curve, and scientists usually try to work within the “linear portion” of the curve.
In practice, variations in the sample matrix, analyte concentrations, or other conditions require some trial and error to see which fit returns the best R2 value, as a linear fit is not always the best option.
For ASTM D7511, calibration curve fitting can be 1st order (linear), 2nd order (parabolic), or weighted linear. Weighted linear requires a minimum of three replicates for each calibrant. We suggest selecting the 1st order curve fit for the 0-100 ppb range. It is the simplest representation between analytical signal and concentration. For those who would like an expanded calibration range beyond 100 ppb, we recommend the 2nd order calibration fit. These three options allow the analyst to select the best curve fit for the sample matrix and analytical range.

The OI FS3700 is a multi-parameter benchtop instrument that measures several critical parameters like cyanide, ammonia, nitrate, nitrite, phosphorous, and more.

The ASTM D7511 Method offers several options of reagent combinations, and it is important to note that expired reagents can cause disrupted flow, potentially damaging the UV Teflon coil used in the system.
Total Acid 1 (TA1) and Total Acid 2 (TA2) reagents are only stable for two weeks after the hypophosphorous acid has been added. When TA1/hypophosphorous acid solution degrades, the reduction capacity of the reaction declines, causing Fe from FeCN complexes to electrostatically bond to the walls, as well as tailing of peaks that contain FeCN; consequently generating low FeCN recoveries.
Expired NaOH reagent will give low cyanide recoveries and split peaks. Low cyanide recoveries result from weak NaOH, leading to poor capture and disorganized transport of diffused cyanides to the flow cell. This will be evident by two concentration gradients for the same peak (i.e., split peak).


Samples containing particulate matter or debris will obstruct the FIA valve, creating backpressure and compromising the detector's bassline. We recommend filtering these types of samples with a Whatman .40 µm filter prior to analysis.

Routine maintenance of an automated chemistry analyzer, including the chemistry channels being used, is incredibly important for achieving reliable and successful operation. This is no different for cyanide chemistry channels, which require close attention due to their complexity.
For instance, if you run ~200 samples a week, you should be prepared to replace reagent tubing weekly. Tend to the mixing tees, air tees, and Teflon coil as needed. The 312 nm UV lamp should be replaced every six months to ensure complete dissociation of strong metal-cyanide complexes. For a complete maintenance guide, view chapter 7 of the FS3700 manual.
Top 5 Tips for Cyanide Analysis
- Choose the right cyanide measurement method
- Select the right curve
- Only use high-quality reagents
- Careful sample treatment and preservation
- Maintain cyanide cartridge components
For more information, visit our cyanide parameter page.
We hope these tips from our experts will help you get years of great data from your system!
