 Introduction
Introduction
There has been an increase in concern for detecting and removing organic chloride species from crude aromatic, naphtha, and other hydrocarbon streams. One process called catalytic reforming uses organic chloride to condition the catalyst, which results in low-level chloride contamination downstream. This can take the form of hydrogen chloride (HCl) and organic chlorides. The chloride species can cause several problems in the various refining processes, including forming and depositing ammonium chloride, corrosion, poisoning of catalysts, and fouling of products.1 Since reformates and other aromatic and naphtha streams are used to make gasoline blending stock, aromatic bulk chemicals, and raw materials for plastics, determining and removing contaminants is essential. Chlorides can also be introduced by sample handling and equipment degreasers, so identifying the specific chlorinated species is essential to pinpoint the source of the contamination. Analysis by the halogen-specific detector (XSD) can be helpful in the detection of organic chlorides. This detector offers advantages over other halogen selective detectors, such as it contains no radioactive source, doesn't use organic solvents, and is simple to operate. This application note will show a method for organic chloride analysis with a representative compound list with a calibration range of 0.1 to 10 ppm.
Instrumentation and Methodology
The instrumentation used was an Agilent 7890A Gas Chromatograph and OI Analytical 5360A XSD. The XSD operates as a thermionic emission detector optimized for detecting halogen compounds. The detector assembly consists of a ceramic probe with a platinum coil and bead inserted into a high-temperature reactor. The GC column effluent
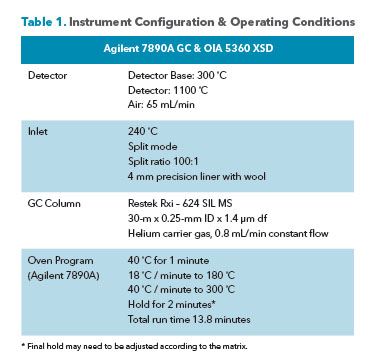
is combusted in an air stream and passed over the bead. The elevated temperature of the reactor causes alkali metal atoms to be released from the ceramic probe and deposited on the bead. The halogenated compounds in the effluent react with the alkali metal atoms on the bead's surface, which increases thermal electron emission. The measured emission current is proportional to the mass of halogen produced. A calibration was analyzed for a representative group of halogenated compounds by manually injecting one μl of standard prepared methanol. Instrument conditions are shown in Table 1.
 Results & Discussion
Results & Discussion
Calibration
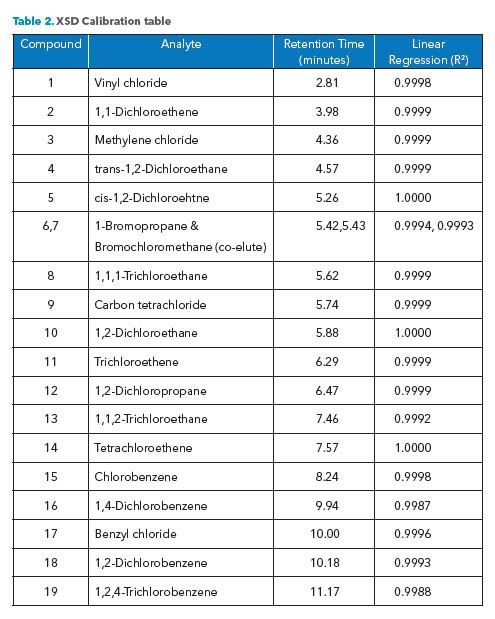
A six-point calibration was performed, which included the range of 0.1 ppm to 10 ppm for all compounds except for 1-Bromopropane. The calibration for this compound was 1.0 ppm to 100 ppm. The response for a brominated compound is approximately ten times less than that of a chlorinated compound on the XSD. The Agilent Chemstation OpenLab software generated calibration curves using linear regression. Please see Table 2.
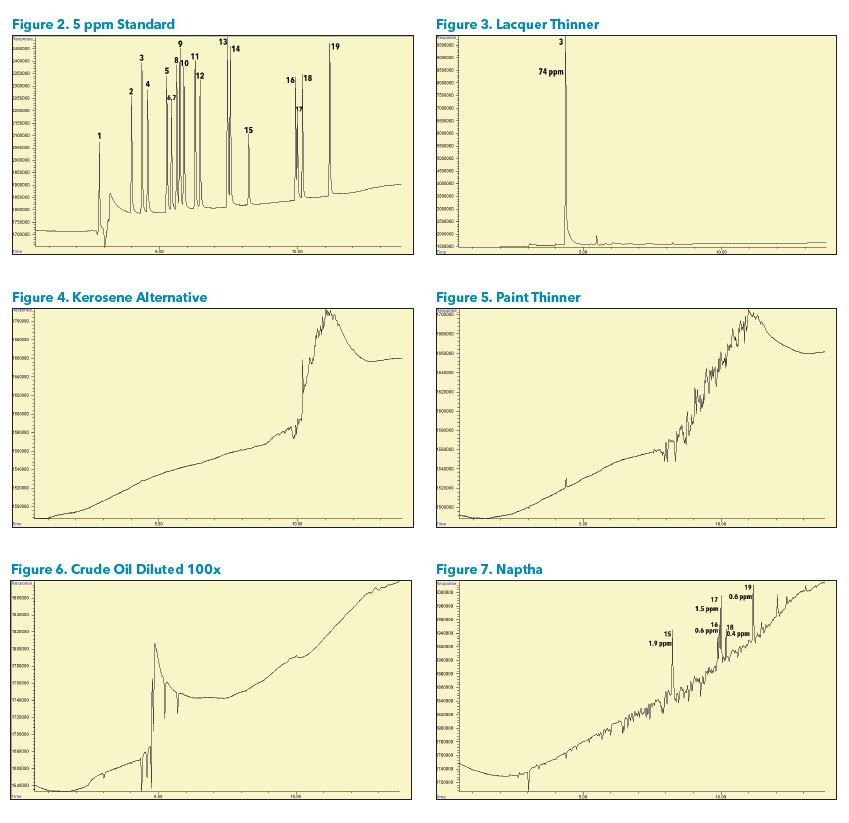
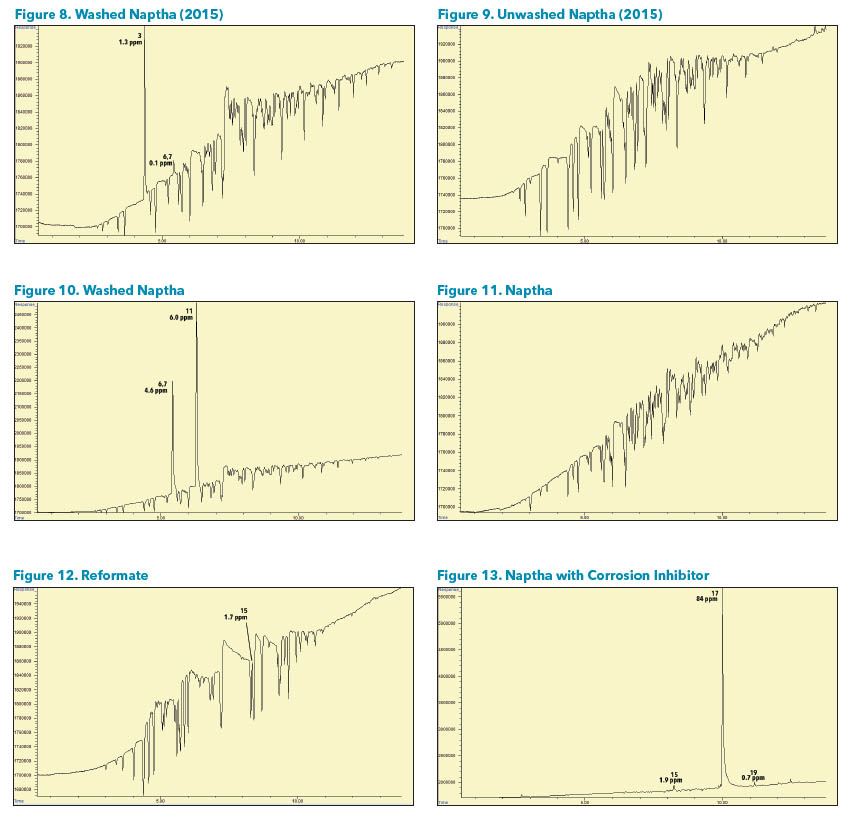
Sample Analysis A variety of petrochemical samples were analyzed. Some samples had very high hydrocarbon concentrations, such as naphtha, which caused dips in the baseline in locations where the hydrocarbons eluted. It may be necessary to dilute or run some samples at a higher split to avoid this effect if interference with a chlorinated compound is suspected.
Some samples contained solvents such as Methylene chloride, 1- Bromopropane, and Trichloroethene that may come from sample handling or the catalytic reforming process. Benzyl chloride may come from corrosion inhibitors or a reaction of a chlorinated compound with Benzene and Toluene. (Figures 2-13).
Conclusions
Refineries have become more aware of the need to detect and remove chlorinated species from their refinery streams. A critical factor in eliminating the chlorides is detecting them in samples.2 Many refineries use gas tube detection methodologies which are not very effective at detecting low chloride concentrations.3 The XSD provides data that may help the refineries accurately measure and pinpoint the source of chlorinated compounds in their streams. The presented method is fast, easy to use, and utilizes an easy-to-maintain detector.
References
-
Digitalrefining.com, Removal of Chloride Compounds, April 2003.
-
Digitalrefining.com, Chloride Removal in Refineries, March 2011.
-
Digitalrefining.com, Close Guard Against Chloride Issues, August 2017.
Conclusions
Thank you to Restek for the analytical column used for this work.